 Not to be outdone by defendants Ambry Genetics and Gene-by-Gene's 109-page brief in opposition to Myriad's Preliminary Injunction Motion, Myriad has now filed a Reply Brief weighing in at 140 pages (page limits in briefs seemingly unknown in the District of Utah). The length is perhaps unsurprising, in view of the expansiveness of the arguments raised for and against a preliminary injunction in this case, as well as the still-settling state of the law regarding claims to genetic diagnostic methods.
Not to be outdone by defendants Ambry Genetics and Gene-by-Gene's 109-page brief in opposition to Myriad's Preliminary Injunction Motion, Myriad has now filed a Reply Brief weighing in at 140 pages (page limits in briefs seemingly unknown in the District of Utah). The length is perhaps unsurprising, in view of the expansiveness of the arguments raised for and against a preliminary injunction in this case, as well as the still-settling state of the law regarding claims to genetic diagnostic methods.
The brief is structured according to the requirements for grant of a preliminary injunction, Myriad contending that it is likely to prevail on the merits (this argument taking up the bulk of the brief), that it will suffer irreparable harm, and that the balance of the hardships and public interest favor entry of the injunction. As in the earlier briefs from parties on both sides of the "v", this brief sets the stage with a section extoling the virtues of Myriad's BRCA tests, citing contemporaneous news broadcasts and newspaper stories concerning the discovery of the BRCA genes and Myriad's successful efforts (both scientific and financial) to turn that discovery into a useful test that has saved millions of women from breast and ovarian cancer. That effort is described in the brief as "arduous, long and expensive" and the brief contrasts this history with defendants' allegations that Myriad's development of a genetic diagnostic test for the BRCA genes is merely a "law of nature" using known and obvious techniques. Myriad asserts that these efforts entitle it to the twenty-year term provided for U.S. patents, a term Myriad believes it fully deserves.
The argument that Myriad will prevail on the merits is set forth in two parts, one relating to the primer claims and the other to the method claims. Myriad starts by reminding the court that it need only prevail on one of its asserted claims, Astrazeneca LP v. Apotex, Inc., 633 F.3d 1042, 1050 (Fed. Cir. 2010), or as the brief states in the alternative, Myriad is not entitled to a preliminary injunction only if defendants can convince the court that "no single patent claim is patent-eligible, infringed and valid." According to Myriad, the specificity and narrow scope of the asserted claims makes defendants' task "even more difficult," and ultimately defendants must fail.
Regarding the composition claims, to probes and primers used in the diagnostic methods, Myriad reiterates its earlier argument that the Supreme Court in Association for Molecular Pathology v. Myriad Genetics, Inc., 133 S.Ct. 2107 (2013) defined "a continuum" of patent eligibility wherein genomic DNA was not eligible but "synthetic" DNA is (the claimed probes and primers being such synthetic DNA):
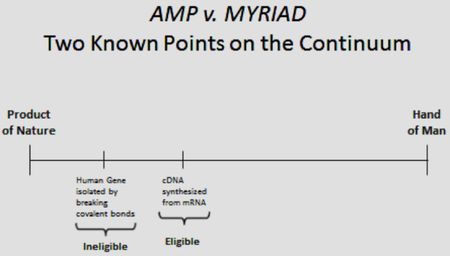 According to Myriad, the Court's decision in the Myriad case was narrow: "We merely hold that genes and the information they encode are not patent eligible under §101 simply because they have been isolated form the surrounding genetic material." Id. at 2120 (emphasis added). Myriad reiterates arguments it raised before the Court in the Myriad case, that the probes and primers are "distinct" from naturally occurring DNA and possess a novel utility resulting from human manipulation. Myriad disputes defendants' argument that the Court categorically prohibited patenting oligonucleotides as an overbroad misreading of the Myriad decision. According to Myriad, the Court set up a dichotomy between DNA that was "merely" isolated from the chromosome and DNA that was "synthetically created." Myriad relies upon the Court's determination that claims 1 and 5 (encompassing genomic DNA encoding the BCRA 1 gene and oligonucleotide fragments thereof, respectively) of U.S. Patent No. 5,747,282 were patent-ineligible, while claims 2 and 6 (encompassing cDNA encoding the BCRA 1 gene and oligonucleotide fragments thereof, respectively) were patent eligible. Myriad contends that it was thus "not the nature of the nucleotide sequence that determines whether a particular DNA molecule is patentable, but rather the extent to which the molecule constitutes a 'naturally occurring' compound versus one created in a laboratory" because "in framing the issue, the Court expressly noted that cDNA shares the 'same protein-coding information' with a segment of natural DNA from the same gene. Id. at 2111." The crux of this portion of Myriad's argument can be found in this quotation from the Supreme Court's Myriad decision:
According to Myriad, the Court's decision in the Myriad case was narrow: "We merely hold that genes and the information they encode are not patent eligible under §101 simply because they have been isolated form the surrounding genetic material." Id. at 2120 (emphasis added). Myriad reiterates arguments it raised before the Court in the Myriad case, that the probes and primers are "distinct" from naturally occurring DNA and possess a novel utility resulting from human manipulation. Myriad disputes defendants' argument that the Court categorically prohibited patenting oligonucleotides as an overbroad misreading of the Myriad decision. According to Myriad, the Court set up a dichotomy between DNA that was "merely" isolated from the chromosome and DNA that was "synthetically created." Myriad relies upon the Court's determination that claims 1 and 5 (encompassing genomic DNA encoding the BCRA 1 gene and oligonucleotide fragments thereof, respectively) of U.S. Patent No. 5,747,282 were patent-ineligible, while claims 2 and 6 (encompassing cDNA encoding the BCRA 1 gene and oligonucleotide fragments thereof, respectively) were patent eligible. Myriad contends that it was thus "not the nature of the nucleotide sequence that determines whether a particular DNA molecule is patentable, but rather the extent to which the molecule constitutes a 'naturally occurring' compound versus one created in a laboratory" because "in framing the issue, the Court expressly noted that cDNA shares the 'same protein-coding information' with a segment of natural DNA from the same gene. Id. at 2111." The crux of this portion of Myriad's argument can be found in this quotation from the Supreme Court's Myriad decision:
Scientists can . . . extract DNA from cells using well known laboratory methods. These methods allow scientists to isolate specific segments of DNA—for instance, a particular gene or part of a gene—which can then be further studied, manipulated, or used. It is also possible to create DNA synthetically through processes similarly well known in the field of genetics. One such method begins with an mRNA molecule and uses the natural bonding properties of nucleotides to create a new, synthetic DNA molecule. . . . This synthetic DNA created in the laboratory from mRNA is known as complementary DNA (cDNA). Id. at 2112.
Myriad expands on this interpretation of the Court's Myriad decision, stating that the Court "recognized that, in contrast to naturally occurring DNA that may be 'extracted from cells using well known laboratory methods,' '[i]t is also possible to create DNA synthetically through processes similarly well known in the field of genetics," going on to discuss only one such method, i.e., cDNA synthesis. However, Myriad argues that "the Court expressly recognized that there are several ways of creating synthetic DNA of which the creation of cDNA was only " a particular one, concluding that "[t]he Court thus at least implicitly ruled that other forms of synthetic DNA are also patentable." This interpretation is also consistent with dicta in the opinion relating to an "application of knowledge of the BRCA sequence[s]," recited with apparent approval in Section III of the Myriad opinion. Myriad also argues that what are claimed here are pairs of oligonucleotide primers, which are "specially designed and made by the hand of man in a laboratory" as well as being "markedly distinct both in form and function from naturally occurring DNA." Because "even a single primer molecule would qualify as patent eligible under AMP" "[a] fortiori, a functionally-coordinated pair of single-stranded DNA primers capable of synthesizing a new DNA molecule having a sequence in common with a specific portion of the sequence of BRCA1 or BRCA2 genes manifestly does not occur in nature and is patent eligible" (emphasis in the brief). Myriad extends this distinction between naturally occurring DNA extracted from a cell and synthetic primers and probes with the following argument:
Some of Myriad's claims in AMP were held invalid because some of the compositions encompassed by those claims originated in the human body, and the sole basis for claiming them was merely separating them from their natural origins by breaking of covalent bonds. cDNA and primers are fundamentally different because they originate not in the human body, but in a laboratory. In fact, primers originate in the head of the scientist designing them. To be sure, the scientist utilizes his knowledge of the genetic sequences, chemistry, and the complementarity of DNA bases in designing a particular pair of primers, just as any inventor makes use of natural laws and natural building blocks in creating any invention. For example, a researcher creating a new pharmaceutical molecule will design it to be complementary to bind to a receptor within the body based on natural laws and building blocks. But when a scientist designs a pair of primers and imbues them with the coordinated chemical properties needed to carry out a specific chemical reaction, the scientist performs the hallmark of "invention" in that he literally conceives of brand new molecules. Their genesis is not in extracting a pre-existing natural molecule from its surroundings, but instead in reducing this conceived idea to practice by building ("synthesizing") a completely new molecule where none existed before.
Myriad disparages defendants' characterization of the Court's Myriad opinion as being contrary to the analytical framework set forth in the Myriad opinion as well as imposing a "broad and sweeping effect" that the Court expressly stated it "refrained from making such [a] far-reaching holding[]." Myriad takes advantage of defendants' assertions that "the information (sequence) in DNA was the key" to the Court's decision by making the counter-argument that the actual distinction was between naturally occurring and synthetic DNA, relying on the Court's recognition that while the relevant sequence information between genomic and cDNA was the same, nevertheless cDNA was patent-eligible because it was synthesized by a scientist in a laboratory.
The Myriad decision did not mandate that genetic diagnostic testing was patent-ineligible according to Myriad, citing those portions of the opinion stating that "[a]s the first party with knowledge of the [BRCA1 and BRCA2] sequences, Myriad was in an excellent position to claim applications of that knowledge", and that "[m]any of its unchallenged claims are limited to such applications." Id. And Myriad contends that its asserted method claims satisfy the patent eligibility rubrics set down in Mayo Collaborative Services v. Prometheus Laboratories, Inc., 132 S.Ct 1289 (2012), specifically that the claims recite more than "abstract mental steps" or "routine or conventional steps," and that by reciting steps using patentable synthetic DNA primers the claims fall outside the scope of the Mayo decision. It is the specific application of the knowledge of the BRCA gene sequences that provide the lynchpin for this portion of Myriad's argument, which is that their method claims are "directed to specific laboratory processes that utilize knowledge of the BRCA1 and BRCA2 genes to develop previously unknown techniques." It is this specific application of the specific knowledge of the BRCA genes that Myriad contends brings their claims outside the ambit of the patent ineligibility rationale set out in the Court's Mayo decision, as well as the use of a primer pair Myriad contends is itself patent-eligible for the reasons discussed above. Its methods cannot be considered "routine and well understood," according to Myriad because, in contrast to Prometheus's claims here Myriad "discovered a brand new biomarker (the BRCA1 and BRCA2 genes) in a new indication (hereditary breast and ovarian cancer)" and "was also the first to apply its discovery to design specific applications of generalized techniques such as PCR and DNA sequencing [as well as BCRA gene-specific hybridization] to develop new assays for this new biomarker." As a consequence, Myriad argues, "[t]his case presents opposite facts and compels the opposite conclusion," the critical distinction being the absence of the knowledge of the BRCA genes in the prior art making any assay involving these genes not "routine conventional and well-understood" regardless of how conventional the underlying processes may have been (and in a separate portion of the brief, Myriad includes the use of the probes and primers as further rendering the asserted method claims to be new and not routine).
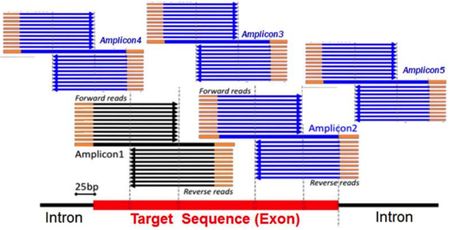
Myriad also argued that it was likely to be able to prove infringement. According to its Reply brief, defendants' argument fail because they are based on erroneous claim construction, fail to address Myriad's infringement arguments, are not "well founded" or "lack merit." Regarding the claim construction allegations, Myriad's arguments are based on defendants' position that including "adaptor," "bar code" or "tag" sequences in their primers place these primers outside the scope of Myriad's claims because the primers are this not "wholly derived" from the native BRCA genes. Myriad argues that this construction of the term "derived from" (to require "wholly" derived from) is contrary to the plain meaning of the term as well as how the phrase "derived from" is used in the specification (which, according to Myriad, include embodiments wherein the primers comprise adaptor, bar code and tag sequences). And Myriad illustrates the deficiencies of defendants' arguments that its amplicons contain intro-derived (and this non-cDNA based) sequence, using an illustration from Ambry's own declarant showing that some of the amplicons are completely comprised of exonic (i.e., cDNA) sequences:
 Turning to defendants' invalidity arguments based on the prior art, Myriad argues that Ambry and Gene-by-Gene have not "raised a substantial question of invalidity" (the preliminary injunction-defeating standard) with regard to either anticipation (over the Schutte reference) or obviousness. According to Myriad, the Schutte reference isn't prior art (being published after Myriad's priority date), nor does it disclose primers capable of amplifying BRCA genomic DNA. Moreover, the other cited prior art does not disclose "a pair of single stranded DNA primers" specific for the human BRCA genes when disclosing double-stranded DNA fragments encompassing such primer sequences, and the experimental efforts disclosed in these references did not amount to prior invention under §102(g). Myriad asserts that it had conceived of the invention(s) recited in their asserted claims prior to the earliest dates defendants assert and thus that the claims are not anticipated. And regarding the BRCA2 primer claims, Myriad argues that defendants read out of the claims the limitation that these primers are used to amplify BRCA2-specific DNA, something not disclosed in the cited reference.
Turning to defendants' invalidity arguments based on the prior art, Myriad argues that Ambry and Gene-by-Gene have not "raised a substantial question of invalidity" (the preliminary injunction-defeating standard) with regard to either anticipation (over the Schutte reference) or obviousness. According to Myriad, the Schutte reference isn't prior art (being published after Myriad's priority date), nor does it disclose primers capable of amplifying BRCA genomic DNA. Moreover, the other cited prior art does not disclose "a pair of single stranded DNA primers" specific for the human BRCA genes when disclosing double-stranded DNA fragments encompassing such primer sequences, and the experimental efforts disclosed in these references did not amount to prior invention under §102(g). Myriad asserts that it had conceived of the invention(s) recited in their asserted claims prior to the earliest dates defendants assert and thus that the claims are not anticipated. And regarding the BRCA2 primer claims, Myriad argues that defendants read out of the claims the limitation that these primers are used to amplify BRCA2-specific DNA, something not disclosed in the cited reference.
Nor does the cited art render the asserted claims obvious according to Myriad's arguments. The reason: discovery of the BRCA genes was not obvious, inter alia, because there were not a "finite number of predictable solutions" leading to the elucidation of the BCRA genes, nor was there any reasonable expectation of success that the BCRA genes would be isolated and identified. Myriad concedes that "[a]t the time of the invention, those of skill in the art had determined that the human BRCA1 gene was likely responsible for a large proportion of familial breast and ovarian cancer cases" and that "[i]t was also understood that the BRCA1 gene was linked to chromosome 17q21." However Myriad contends that the absence of any knowledge of the "biochemical effects underlying inherited human breast cancer" precluded knowledge of the protein product of the cognate gene and necessitated "positional cloning" efforts for the BRCA1 gene's isolation. This uncertainty was reinforced by contemporaneous reports that the BRCA1 gene could be found in genomic DNA regions from 4-18 million basepairs in length. This confusion is illustrated by the following graphic in the brief showing where the BCRA1 gene might have been located in view of what was known in the art at the time Myriad successfully cloned the gene:
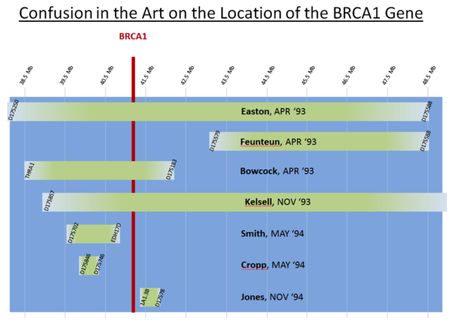 Defendants error, according to Myriad, is that they:
Defendants error, according to Myriad, is that they:
[I]ncorrectly assert that a person of ordinary skill in the art would immediately be drawn to the ranges identified by the Bowcock and Kelsell articles instead of the ranges identified by the other references, several of which post-date Bowcock and Kelsell. In selecting a region to search for the BRCA1 gene, the ordinarily skilled artisan could have selected any of the above regions, most of which we now know would have led (and for many did lead) to a dead end in that search. In ignoring these references, Defendants focus on the region where the BRCA1 gene was ultimately discovered (even though many skilled artisans at the time did not) for no apparent reason other than they now know (because of Myriad's discovery) that the BRCA1 gene is located in that region. The Federal Circuit has routinely warned against such an approach. See Mintz v. Dietz & Watson, Inc., 679 F.3d 1372, 1379 (Fed. Cir. 2012) [and] Otsuka Pharm. Co. v. Sandoz, Inc., 678 F.3d 1280, 1296 (Fed. Cir. 2012) (citations to the record omitted)
In other words, hindsight reconstruction according to Myriad. And there were other candidate genes that others in the field had already incorrectly identified as the responsible locus, thus further reinforcing the non-obviousness of Myriad's method claims. The brief then sets out the difficulties actually experienced by the Myriad inventors, and others, to support its conclusion that "Defendants' claim that 'by the start of 1994, it was clear to the field what steps and techniques would be employed in order to discover the location and sequence of the BRCA1 gene' is meritless."
In addition, and particularly with regard to the BRCA2 gene Myriad argues that the inventors followed "an unconventional path" to identify the gene, and in fact argued that one of the cited references, the Wooster (1995) reference, "shows exactly why the discovery of the BRCA 2 gene was not obvious," i.e. the purported BRCA2 gene contained a large sequence of unrelated DNA at the position encoding the purported carboxyl terminus of the gene. Finally, Myriad asserts that the objective indicia support its argument that defendants have not shown obviousness by clear and convincing evidence, including evidence of "i) whether there was a long-felt need for the claimed invention, (ii) failure of others to achieve the invention, (iii) industry praise and respect for the claimed invention; (iv) unexpected results from the claimed invention, (v) skepticism of others; and (vi) the commercial success of the claimed invention."
More briefly Myriad addresses defendants' arguments regarding written description and indefiniteness, particularly with regard to whether the terms "BRCA1 gene" and "BRCA2 gene" include cDNA embodiments thereof.
As for the other requirements for granting a preliminary injunction, Myriad asserts irreparable harm from price erosion that cannot be "readily compensated," as well as damage of reputational harm. Comparing the damage to Myriad with the loss of the "headstart" advantage defendants argued they would suffer balances in favor of Myriad (according to Myriad) and the public interest is satisfied by Myriad's satisfaction of the market for hereditary predisposition testing for BRCA gene–related diagnostic testing. Myriad contests the defendants' allegations that their testing is inadequate, that Myriad does not afford "superior access and affordability," facts Myriad states defendants "cannot dispute," and that the policy-based allegations regarding the public interest are irrelevant and "ignore Myriad's extensive contributions to the market."
The brief also includes a recitation of facts Myriad disputes, including defendants' allegations that the primer and probe sequences are equivalent to the sequence information they encode (where Myriad reminds the court that DNA is not information like computer storage media), and that the claimed primers were indistinguishable from DNA as it exists in the chromosomes (Myriad asserting that "a segment of synthetically made DNA creates a different chemical compound than exists in the "natural" DNA in the human chromosomes because they both consist of a different overall collection of molecules. Different collections of molecules create different chemical compounds with different properties; all these factual assertions rely upon expert declarations). Myriad also contests defendants' arguments contained in their factual allegations that PCR, DNA sequencing and DNA hybridization are "routine and conventional," asserting in response that while these techniques had been applied to "certain" genes prior to August 14 1994 (Myriad's earliest priority date), their application to the human BRCA genes was neither well-known nor even possible.
The Myriad case is not over, no matter how the district court rules, and it can be expected that the complete contours of the determination about patent-eligibility and patentability of genetic diagnostic testing will continue for the foreseeable future.