On October 10, 2024, the FDA approved Accord BioPharma’s Imuldosa™ (ustekinumab-srlf), the fifth biosimilar of Janssen / Johnson & Johnson’s Stelara® (ustekinumab). Imuldosa™ was developed by Dong-A ST in collaboration with Meij Seika Pharma, and Accord is responsible for commercialization in the U.S. Under an October 2023 settlement agreement, Imuldosa™ can launch in the U.S. beginning no later than May 15, 2025.
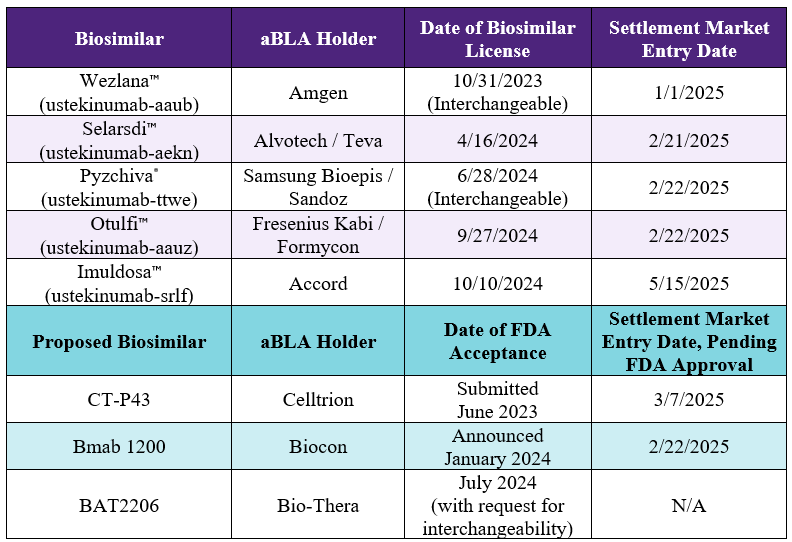
The table below shows the other approved and pending aBLAs for Stelara® biosimilars as well as their settlement market entry dates. There are no pending patent disputes related to Stelara® biosimilars.
Stelara® was chosen as one of the first 10 drugs for Medicare price negotiations under the Inflation Reduction Act. In August 2024, the Centers for Medicare & Medicaid Services (CMS) announced that as a result of the price negotiations, beginning January 1, 2026, under Medicare Part D, a 30-day supply of Stelara® will cost $4,695, a 66% discount from its 2023 list price of $13,836. However, the anticipated launch of Stelara® biosimilars in early 2025 may affect the status of Stelara® on Medicare’s price negotiation list.
Johnson & Johnson reported Stelara® U.S. sales of $6.97B in 2023.
_____________________________________________________
The author would like to thank April Breyer Menon for her contributions to this article.