
The Federal Circuit recently reviewed yet another decision by the Patent Trial and Appeal Board (PTAB) of the U.S. Patent and Trademark Office in Amerigen Pharmaceuticals Ltd. v. UCB Pharma GmbH, and once again reviewed whether a failed petitioner in an inter partes review (IPR) proceeding had standing to appeal an adverse judgment.
The issues arose in an IPR by Petitioner Amerigen involving claims 1-5 and 21-24 of U.S. Patent No. 6,858,650, which the PTAB found to be non-obvious over the art cited in the petition. The challenged claims of the '650 patent are directed to prodrug forms of 3,3-diphenylpropylamines, notably fesoterpdone:
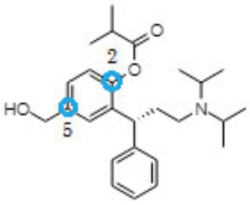
marketed by UCB as Tovoaz® for treating urinary incontinence. The positions noted by blue circles in the depiction of the chemical compound shown above are the sites where the chemical has been (the 2-position) or could be (the 5-position) derivatized to form a prodrug, defined as an inactive form of the drug that must metabolized after administration to a patient for the drug to have a biological effect. The active compound, 5-hydroxymethyl tolterodine, is itself a metabolite of an older drug, tolterodine which is sold as Detrol® for anti-urinary incontinence effects:

Fesoterodine, in contrast, differs from 5-HMT in having an isobutyryl ester in place of the hydroxyl group at the 2-position. This modification raised the core issue regarding patentability before the PTAB and the Federal Circuit: would it have been obvious to make this substitution to create an inactive prodrug requiring metabolic conversion to product the active 5-HMT in vivo?
The Petitioner asserted two grounds of obviousness: the first, over the combination of the Detrol® label; a scientific publication to Postlind et al. disclosing metabolism of tolterodine to 5-HMT; a treatise disclosing design of prodrugs (Bungaard); a PCT application naming Bungaard as an inventor (the '459 PCT): a separate scientific publication to Byrnne et al. further directed to metabolism of tolterodine to 5-HMT; and another scientific publication to Berge et al., disclosing preparation of pharmaceutical salts. The second asserted obviousness ground combined Byrnne, Bungaard, '459 PCT, and another PCT application to Johansson (the '337 PCT). The PTAB found that neither of these asserted combinations satisfied Petitioner's burden of showing the '650 patent claim were obvious, and Amerigen appealed.
The Federal Circuit affirmed, in an opinion by Judge Lourie joined by Judges Chen and Stoll. Before reaching the substantive patentability issues, the panel first addressed argument from UCB that Amerigen did not have standing to pursue the appeal. This argument was based on a decision in ANDA litigation that Amerigen failed to show the '650 patent was invalid and thus the FDA would not approve Amerigen's ANDA for a generic version of Tovoaz® until the '650 patent expired. As a consequence, according to UCB's argument, Amerigen could not be subject to infringement liability and thus could not suffer injury sufficient to support standing in the appeal. Amerigen countered by noting that invalidation of the '650 patent by the Federal Circuit reversing the PTAB would "advance the launch of its product" and that the continued viability of the '650 patent created a concrete injury sufficient to confer standing under Article III.
The opinion recites the canonical requirements for standing (an appellant must have "(1) suffered an injury in fact, (2) that is fairly traceable to the challenged conduct of the defendant, and (3) that is likely to be redressed by a favorable judicial decision," citing Spokeo, Inc. v. Robins, 136 S. Ct. 1540, 1547 (2016)) and held that Amerigen satisfied these requirements. It was not disputed that launch of Amerigen's generic product was blocked by the continued vitality of the '650 patent and that a decision invalidating the patent (by the PTAB or the Court) would "advance [the] drug's launch." The opinion explains that the '650 patent is listed in the FDA's "Orange Book" and that UCB would have the obligation of delisting this patent if the Court reversed the Board's decision in the IPR. In that case, the predicate basis for Amerigen's Paragraph III certification would be lifted and Amerigen would be able to begin marketing its drug upon expiration of UCB's other listed patents; this will occur about three years before the expiration date of the '650 patent. This constitutes a "concrete, economic interest" in Amerigen selling its tentatively approved drug (wherein all approval requirements except UCB's patent have been satisfied) according to the opinion. The panel cited Apotex, Inc. v. Daiichi Sankyo, Inc., 781 F.3d 1356, 1359–61 (Fed. Cir. 2015), in support of its decision here, where the Court had held that listing of a patent in the Orange Book could create a controversy "of sufficient immediacy and reality" to satisfy Article III standing requirements. The opinion did not credit UCB's argument that the issue was controlled by the Federal Circuit's decision in Janssen Pharmaceutica, N.V. v. Apotex, Inc., 540 F.3d 1353 (Fed. Cir. 2008), noting that the Janssen decision involved considerations relating to the Hatch-Waxman Act that were not shared by the IPR provisions of the America Invents Act (which provided much more broadly for parties to challenge granted U.S. patents).
Turning to the substantive grounds of the PTAB's decision that Amerigen did not satisfy its burden of showing the challenged claims of the '650 patent were obviousness, the Federal Circuit parsed these references into three general categories: first (the Detrol Label, Postlind, and Brynne), those references related to tolterodine, its metabolism, and pharmacokinetics; second (Bundgaard and the '459 PCT), references related to designing prodrugs; and third (Berge and the '337 PCT), related to pharmaceutical salts. The first set of references, according to the Federal Circuit, taught that there was a subset of the patient populations that were poor metabolizers and that did not effectively activate tolterodine to 5-HMT, but that this distinction did not particularly reduce the efficacy of the drug. The second set, in discussing generally prodrugs and their production, discussed the use of esters to as "common prodrug substituents." Finally, the third set of references discussed pharmaceutical salts and particularly fumarate salts like fesoterodine. The panel noted that the PTAB found that one having ordinary skill in the art would have chosen 5-HMT as a lead compound for further development. But based on expert testimony the Board found that the skilled worker would not have modified 5-HMT by esterifying the 2-position hydroxyl group with isobutyrate. This decision was based on disagreement between the parties (and their experts) regarding whether 5-HMT was not sufficiently lipophilic and thus would have decreased bioavailability. One basis upon which the PTAB credited Patent Owner UCB's expert over Amerigen's expert on this issue was that UCB's expert applied the "Rule of 5" found in a prior art reference directing that a skilled worker assess bioavailability under these criteria:
If:
(1) there are more than 5 hydrogen-bond donors;
(2) there are more than 10 hydrogen-bond acceptors;
(3) the molecular weight is greater than 500; and
(4) the calculated log P is greater than 5
then a compound can be expected to have a "bioavailability problem." 5-HMT did not evince expected reduced bioavailability using the Rule of 5, and thus the PTAB credited UCB's expert's testimony on this issue (which Amerigen's expert did not rebut).
Having made this determination, the Board held that a skilled person would not have esterified the 2-position of 5-HMT to solve a bioavailability problem that she would not recognize existed in the prior art. In addition, Petitioner had not produced evidence that 5-HMT esterified at the 2-position would be inactive (i.e., actually be a prodrug). The Board also found that even if a skilled worker would have been motivated to modify 5-HMT, expert testimony supported a conclusion that producing such a prodrug would not have been "a matter of routine optimization," at least in part due to the "many possible molecular modifications of 5-HMT consistent with a prodrug design." While there were a number of possible embodiments of esterified 5-HMT (including esterifying the hydroxyl group at the 5-position), even if the universe of esters were limited to alkyl esters of six carbons or fewer at the 2-position there would be 86 possible compounds and testing each one would not have been routine.
In affirming the PTAB's decision the Federal Circuit noted its limited basis for review regarding the Board's factual determinations under In re Gartside, 203 F.3d 1305, 1316 (Fed. Cir. 2000). Almost the entirety of the panel's decision focused on the substantial evidence relied upon by the Board in making its nonobviousness determination, culminating in the statement that "[i]t was Amerigen's burden to show that the 'prior art would have suggested making the specific molecular modifications necessary to achieve the claimed invention,'" citing Takeda Chem. Indus., Ltd. v. Alapharm Pty., Ltd., 492 F.3d 1350, 1356 (Fed. Cir. 2007) (emphasis added) (quoting In re Deuel, 51 F.3d 1552, 1558 (Fed. Cir. 1995)). Under the facts before the Court, there was no basis for reversing the PTAB's decision and the panel declined to do so.
Amerigen Pharmaceuticals Ltd. v. UCB Pharma GmbH (Fed. Cir. 2019)
Panel: Circuit Judges Lourie, Chen, and Stoll
Opinion by Circuit Judge Lourie