The Federal Circuit recently applied well-established principles of obviousness in affirming the Patent Trial and Appeals Board's invalidation of several patents related to antifungal formulations in Anacor Pharmaceuticals, Inc. v. Flatwing Pharmaceuticals, LLC.
The subject matter of the claimed invention was a topical formulation of 1,3-dihydro-5-fluoro-1-hydroxy-2,1-benzoxaborole (known as tavaborole):
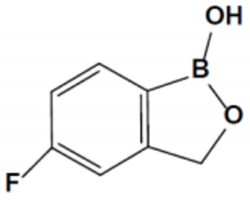
and sold as KERYDIN® for treatment of a fungal infection (onychomycosis) caused by Trichophyton rubrum or T. mentagrophytes. Flatwing Pharmaceuticals successfully initiated inter partes review of all claims of U.S. Patent Nos. 9,549,938 (the "'938 patent"), 9,566,289 (the "'289 patent"), 9,566,290 (the "'290 patent"), and 9,572,823 (the "'823 patent") for being as unpatentable for obviousness; the opinion characterizes dependent claim 2 of the '823 patent as being representative:
1. A method of delivering a compound, in a human, from a dorsal layer of a nail plate to a nail bed to treat onychomycosis caused by Trichophyton rubrum or Trichophyton mentagrophytes, the method comprising: contacting the dorsal layer of the nail plate with a pharmaceutical composition comprising a compound that penetrates the nail plate, the compound being [tavaborole] or a pharmaceutically acceptable salt thereof, thereby treating onychomycosis due to Trichophyton rubrum or Trichophyton mentagrophytes.
2. The method of claim 1, wherein the pharmaceutical composition is in the form of a topical solution comprising 5% w/w of [tavaborole], and wherein the pharmaceutical composition further comprises ethanol and propylene glycol [emphasis added].
(Although other claims in the related patents in inter partes review recite ranges from 1-15%.)
The PTAB had earlier found claims in two related patents to be invalid for obviousness (see "Anacor Pharmaceuticals, Inc. v. Iancu (Fed. Cir. 2018)"). Here, the PTAB applied some of the same art and arrived at the same conclusion of obviousness. The Board decided that the prior art taught the use of antiifungal compounds (albeit not tavaborole) at concentrations overlapping the 5% recited in claim 2 of the '823 patent for treating fungal infections and thus the combination taught all the elements of the claimed invention. The Board held that routine optimization by the skilled worker would have produced the claimed formulations which would have been reasonably expected to be effective against onchomycosis. The Board also rejected Anacor's arguments regarding challenges posed by using organoboron compounds that would have precluded the existence of a reasonable expectation of success, based on expert testimony, and further rejected that the prior art (which taught 10% antifungal concentrations in prior art formulations) taught away from obviousness of the claimed invention teaching 5% tavaborole formulations.
The Federal Circuit affirmed, in an opinion by Judge Lourie joined by Judges O'Malley and Chen. Using the substantial evidence standard for factual questions arising before the PTAB, In re Gartside, 203 F.3d 1305, 1316 (Fed. Cir. 2000), and de novo review of questions of law, the panel agreed with the Board's conclusion that the choice of 5% tavaborole concentrations in the claimed in formulation was a matter of routine optimization. The Federal Circuit recognized that tavaborole concentration was "a result-effective variable[] such that one could optimize nail penetration by routine experimentation within a predictable range of concentrations" in support of its opinion. The evidence showed that screening techniques existed in the art for testing for efficacy. The art also taught a relationship between nail penetration and antifungal effectiveness, for example, wherein the art taught that 5% econazole concentrations were effective for penetrating nails. According to the opinion, because tavaborole is a smaller molecule than econazole the skilled worker would have expected it to be effective in penetrating nails and thus being effective as an antifungal agent in that milieu.
The panel also rejected Anacor's teaching away argument, because the art showed that any diminution of effectiveness caused by lowering econazole concentration from 10% to 5% was modest and depended on other factors such as excipients used in the formulation. The opinion characterized the attitude exhibited by the art as not amounting to discouragement against using 5% econazole formulations and thus concluded that the art did not teach away from them.
Finally, with regard to Anacor's argument that the skilled worker would have recognized that using organoborane compounds posed some difficulties in formulation, the opinion states that "[prior art] describing in vivo inhibition of a common fungus with organoboron compositions—formulated in either mineral oil or petroleum jelly—is especially damaging to Anacor's arguments." Moreover, the Federal Circuit found that any purported challenge was not reflected in the specification, wherein "the specification does not offer any guidance beyond citation of well-known guides to pharmaceutical formulation" for making the claimed organoborane formulations.
Finding no error by the PTAB, the Federal Circuit affirmed its judgment of obviousness for all challenged claims.
Anacor Pharmaceuticals, Inc. v. Flatwing Pharmaceuticals, LLC (Fed. Cir. 2020)
Panel: Circuit Judges Lourie, O'Malley, and Chen
Opinion by Circuit Judge Lourie