[author: Kevin E. Noonan]
 "Genome projects" for a variety of species (comprising a complete sequence determination of a species' genomic DNA) has been underway for the past dozen or so years, in the wake of the completion of the Human Genome Project at the turn of the century. While such a sequence determination remains the "gold standard" for a full appreciation of the gene structure and complement of a species, partial genome sequences, focusing on an animal's protein-coding sequences can also be useful in deriving phylogenetic relationships and understanding how genes are associated with (if not directly responsible for) shared phenotypic characteristics.
"Genome projects" for a variety of species (comprising a complete sequence determination of a species' genomic DNA) has been underway for the past dozen or so years, in the wake of the completion of the Human Genome Project at the turn of the century. While such a sequence determination remains the "gold standard" for a full appreciation of the gene structure and complement of a species, partial genome sequences, focusing on an animal's protein-coding sequences can also be useful in deriving phylogenetic relationships and understanding how genes are associated with (if not directly responsible for) shared phenotypic characteristics.
Such a study was reported recently for the bottlenose dolphin, Tursiops truncates in a paper by Michael McGowen, Lawrence Grossman and Derek Wildman of the Center for Molecular Medicine and Genetics, Wayne State University School of Medicine (Proceedings of the Royal Society: Biological Sciences, 279: 3643-3651). The paper,"Dolphin genome provides evidence for adaptive evolution of nervous system genes and a molecular rate slowdown," reports the results of a comparison of 10,025 protein-coding genes from bottlenose dolphins and orthologous genes from cow (Bovis taurus, the closest living mammalian species in the Order Cetartiodactyla), horse (Equus caballus), dog (Canis familiaris) (together, these four species comprising the laurasiatherian species), mouse (Mus muscalus), elephant (Loxodonta africana), opossum (Monodelphis domestica), platypus (Ornithorhynchus anatinus), chicken (Gallus gallus) and man. The comparison was limited to coding sequences (CDS) ranging from 26335 bp (SYNE1) to 150 bp (C20orf196 and FDPS).
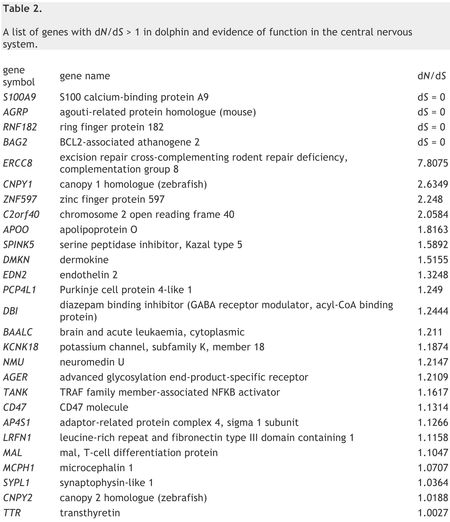
Dolphin genes were found to have the slowest rates of synchronous substitution between the laurasiatherian species and comparable to rates seen in elephants and humans. The mean ratio of the number of non-synonymous substitutions per non-synonymous site to the number of synonymous substitutions per synonymous site (dN/dS) were highest in dolphins of all laurasiatherian species, and again close to human rate ratios. Within the dolphin genome, 228 genes were identified as having (dN/dS) >1 (shown in Table 2), which was interpreted to indicate positive selection at levels (2.26%) significantly higher than seen in other species (horse, 48 or 0.51%; cow, 32 or 0.32%; dog, 11 or 0.12%). Moreover, while at least 46% of genes in all other lineages studied were subject to "purifying selection" (genes having a (dN/dS) <0.1), only 36.4% (or 3646 dolphin genes) were found with this ratio, compared with 44-51% in other species.
Of the 228 dolphin genes (2.26% of total genes sequenced) under positive selection (having dN/dS > 1), 27 of these genes are related to the nervous system including genes homologous to human genes known to be involved in sleep, synaptic plasticity and, when defective, cognitive disability. These include "[s]even genes [that] are identified as being involved in intellectual disabilities and/or microcephaly (ERCC8, AP4S1, MCPH1, TTR), schizophrenia (MAL) or Alzheimer's susceptibility (AGER, RNF182)[; f]ive genes [] involved in neuroendocrine function, neuropeptide hormonal activity, or function as hormones that affect the brain (AGRP, C2orf40, EDN2, NMU, TTR)[; ] genes [that] function in the development of myelin (MAL), neuronal or brain development (CNPY1, ZNF597, PCP4L1, MCPH1), neural potassium channel function (KCNK18), neurite growth (CD47, LRFN1,CNPY2) and synaptic function (BAALC, DBI, SYPL1, AP4S1, LRFN1)." These results were consistent with morphological characteristics of dolphin brains, including "high level of gyrification (cortical folding), expansion of the insular and cingulate cortices, specialized von Economo neurons, high glia to neuron ratio increase in synapse number, reduction of the olfactory system and the large relative size of the cerebral cortex." This suggests to the authors that a genetic analysis might uncover evidence of "convergent evolution" among large-brained mammals. Further, consistent with the source, the authors report that genes "putatively related to cetacean adaptations" were also identified, including "the cardiovascular system (TSPO2, EPGN, PLN, EDN2, PLA2G5, KCNJ2), sperm function and spermatogenesis (TNP1, USP26,SPAG4, NANOS3, SPATA9, CDYL, SOX30, SPATA7, AKAP4, SPATA3, GTSF1), lung development and respiratory function (SCGB3A2, PLUNC, TMPRSS11D), dermal development and function (KRTDAP, SPINK5, SPINK6, IL20, PSORS1C2, DMKN), hair (KRT84), olfaction (OR6B1, OR2AK2), vision (CRYGN), milk (CSN2), glucose and/or glycerol metabolism (OSTN, SOCS6, AQP9), vitamin B-1 and B-12 binding and metabolism (TCN1, THTPA) and a multitude of genes involved in lipid transport and metabolism (APOA2, APOC4, APOO, FABP4, SERINC4, CCDC129, PLA2G5, PNLIPRP3, RARRES2, NR1I3)."
The study also examined metabolism-associated genes (a total of 548 genes), particularly the mitochondrial cellular (genomic) component genes (i.e., genes residing in the cellular chromosome whose gene products localize in the mitochondrion). These genes are believed to be important for energy metabolism. 8.5% of these genes (compared with 1.5-5% for other species) were found to have (dN/dS) > 0.5, including the gene for cytochrome c (CYCS). This is consistent with the increased energy needs of mammals with large brains, it being known that "basal metabolic rates [are correlated] with relative [larger] brain size" [Isler et al., 2006, "Metabolic costs of brain size evolution," Biol. Lett. 2, 557–560] as has been observed in humans [Grossman et al., 2004, "Accelerated evolution of the electron transport chain in anthropoid primates," Trends Genet. 20, 578–585]. These results were consistent with increased brain size, which like in humans is larger than expected when body size is considered.
The authors conclude that their data is consistent with a slower rate of mutation that other species (1.4 x 10-9 substitutions/per site per year compared with an average mammalian mutation rate of 2.2 x 10-9 substitutions/per site per year). These results are also consistent with "adaptive evolution" of both the dolphin nervous system and brain, and although the authors have not established a definitive link between any of the brain-related genes they identified and "morphological or behavioral" traits in dolphins. But they point out that they identified six genes (AP4S1encoding a membrane protein associated with AMPA glutamate receptors; SYPL1encoding a synaptic molecule differentially regulated during human development; LRFN1encoding a synaptic adhesion molecule; BAALC encoding a component of postsynaptic complexes; and DBI encoding a modulator of signal transduction at type A gamma-aminobutyric acid receptors with implications in sleep regulation) that are thought to be involved in synaptic function; similar genes "have been implicated in the evolution of the brain in humans" according to the report.
Genes involved in DNA repair and associated with intellectual disabilities in humans were also identified in the study, including "ERCC8 is a gene involved in transcription-coupled DNA damage repair; mutations in this gene result in Cockayne's syndrome, whose symptoms include microcephaly, neurological defects and premature ageing [Rapin et al., 2006, "Cockayne syndrome in adults: Review with clinical and pathological study of a new case," J. Child Neurol. 21, 991–1006]. ERCC8 shows evidence of positive selection in recent human populations [Voight et al., 2006, "A map of recent positive selection in the human genome," PLoS Biol. 4, e72]. MCPH1 is another gene with evidence of function in DNA damage repair as well as cell cycle regulation. MCPH1 dysfunction can cause microcephaly and has been documented to be under positive selection along the human lineage."
Regarding genes involved in energy metabolism, the positive selection observed by these authors is consistent with an oxygenation rate of 6.0 mL kg/min, greater than in humans or elephants. In this regard the paper makes the following interesting observation:
During the course of primate evolution, metabolism evolved to provide a constant supply of energy to the increasing demands of a larger brain. [Leonard et al., 2003, "Metabolic correlates of hominid brain evolution," Comp. Biochem. Physiol. A 136, 5–15]. Humans have evolved several adaptations, including an increase in visceral fat and the evolution of tissue-specific insulin resistance in the brain that protects nervous tissue from energy shortage; however, malfunctions of this system in humans can cause type 2 diabetes (Kuzawa, 2010 Beyond feast-famine: brain evolution, human life history, and the metabolic syndrome. In Human evolutionary biology (ed. Muehlenbein M.), pp. 518–527. Cambridge, UK: Cambridge University Press). Interestingly, recent research has proposed that dolphins should be seen as a model for type 2 diabetes, as they possess all the hallmarks of the disease [Venn-Watson et al., 2011, "Dolphins as animal models for type 2 diabetes: sustained, post-prandial hyperglycemia and hyperinsulinemia," Gen. Comp. Endocrinol. 170: 193–99)]. Here, we find genomic signatures of adaptive evolution in dolphin genes related to control of food intake such as genes involved in glycerol uptake and/or glucose metabolism (AQP9, OSTN, SOCS6), and a neuropeptide AGRP that is expressed in the hypothalamus and involved in increasing appetite, decreasing metabolism and regulating leptin. In addition, we found several genes under selection related to lipid transport and metabolism [] that may be associated with the large fat reserves found in cetaceans.
This paper is just one example of how elucidating genomic DNA structure can provide insights (including, as set forth above regarding the relationship between energy metabolism and diabetes, unexpected insights) into genotype/phenotype relationships in evolutionarily related species. These results also indicate that the "golden age" of genomics is still, really, in its infancy.