It was not so long ago that many, including members of Congress, were bemoaning the slow approval and introduction into the marketplace of biosimilar alternatives to (generally expensive) biologic drugs. See "Trump Administration Considering Reduction in Biologics Exclusivity Period"; "A Solution in Search of a Problem"; "FDA Issues Plan for Further Facilitating Biosimilar Development"; "Senators Ask FTC to Investigate Biosimilar Litigation Settlement Agreement"; "Biosimilars Action Plan: Balancing Innovation and Competition"; "Congress Jumps on Bandwagon to Reduce Biologic Drug Exclusivity Term". These sentiments were largely from outcome-oriented groups with little understanding or regard for process or an appreciation of what has been required of the U.S. Food and Drug Administration and biosimilar applicants to bring safe and effective biosimilar drugs to market (and to be sure the same chorus would be vocally critical should a biosimilar fail to be safe, effective, and cheaper than the reference biologic drug product). These calls have only intensified in the wake of the COVID pandemic (see, e.g., S6: The Biosimilar Red Tape Elimination Act, introduced on November 17, 2022; "Interchangeable Biosimilars: In a Battle of Safety vs. Cost, Where Does Sen. Lee Stand?").
Things have been looking up with a half dozen biosimilar drugs having been allowed in the past year (see Table). The most recent is Tyruko (natalizumab-sztn) from Sandoz, whose approval was announced by the FDA and is a biosimilar for Biogen's Tysabri (natalizumab) approved for the treatment of relapsing forms of multiple sclerosis (MS), specifically clinically recognized forms of the disease that includes clinically isolated MS, relapsing-remitting MS (West Wing viewers will recognize this as the form President Bartlet suffered from), and active secondary progressive MS.
According to the announcement, in addition to MS the drug is approved for "inducing and maintaining clinical response and remission in adult patients with moderately to severely active Crohn’s Disease (CD) with evidence of inflammation who have had an inadequate response to, or are unable to tolerate, conventional CD therapies and inhibitors of TNF-α (tumor necrosis factor)."
The announcement contains a caution, that use of Tyruko, like Tysabri, is associated with an increased risk of progressive multifocal leukoencephalopathy (PML), "a viral infection of the brain that usually leads to death or severe disability." For this reason availability of the drug is limited by a risk evaluation and mitigation strategy (REMS).
This approval brings to 42 the number of approved biosimilar products.
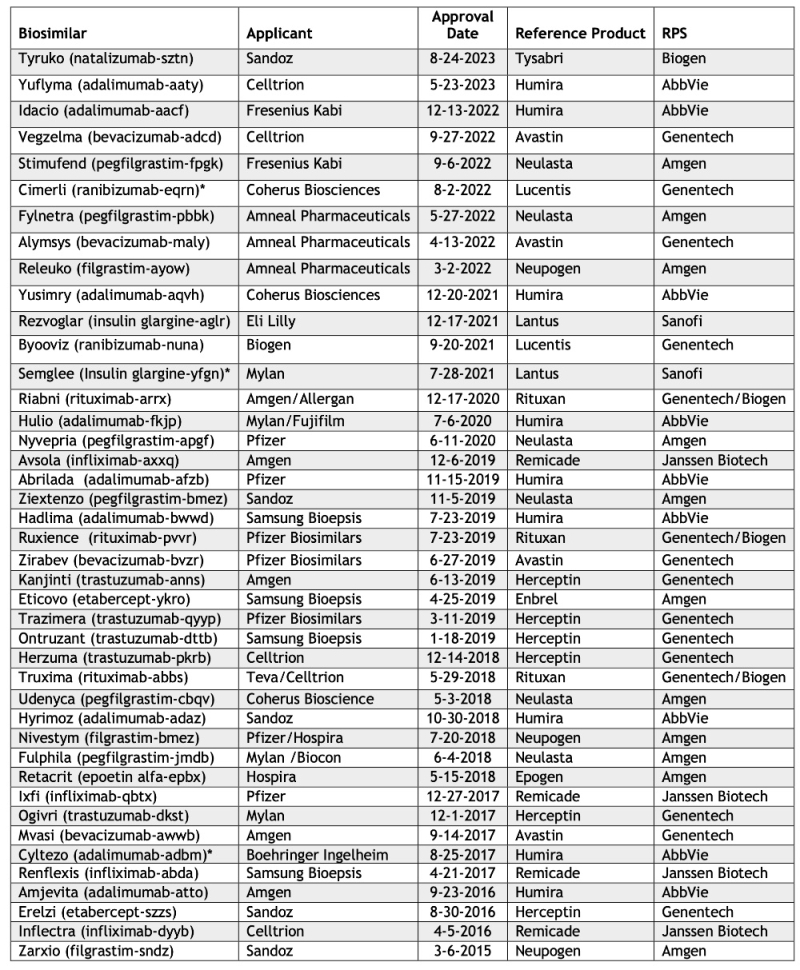 * Approved as interchangeable biosimilar
* Approved as interchangeable biosimilar
[View source.]