On August 13, 2024, the US Court of Appeals for the Federal Circuit held in Allergan v. MSN that “a first-filed, first-issued, later-expiring claim” cannot be invalidated for obviousness-type double patenting (ODP) “by a later-filed, later-issued, earlier-expiring reference claim having a common priority date.” This decision clarifies the scope and applicability of the court’s In re Cellect decision, which held that a patent benefitting from patent term adjustment (PTA) under 35 US Code § 154(b) can be invalidated for ODP over earlier-expiring patents in the same family. The court’s decision is a positive outcome for patentees, ensuring that PTA awards for first-filed, first-issued patents cannot be stripped away by later-issuing child patents that expire earlier.
In this Hatch-Waxman case, Allergan asserted the first patent applied for and issued on eluxadoline, the active ingredient in its Viberzi tablets, against generic manufacturers seeking approval to sell copycat tablets (US Patent No. 7,741,356, referred to below as the “’356 Patent”). The generics argued that the challenged patent, which included 467 days of PTA, was invalid for ODP based on later-filed, later-issued “child” reference patents (referred to below as the “’011 Patent” and the “’709 Patent”). This diagram from the Federal Circuit illustrates the relationship between the challenged patent and the child reference patents:
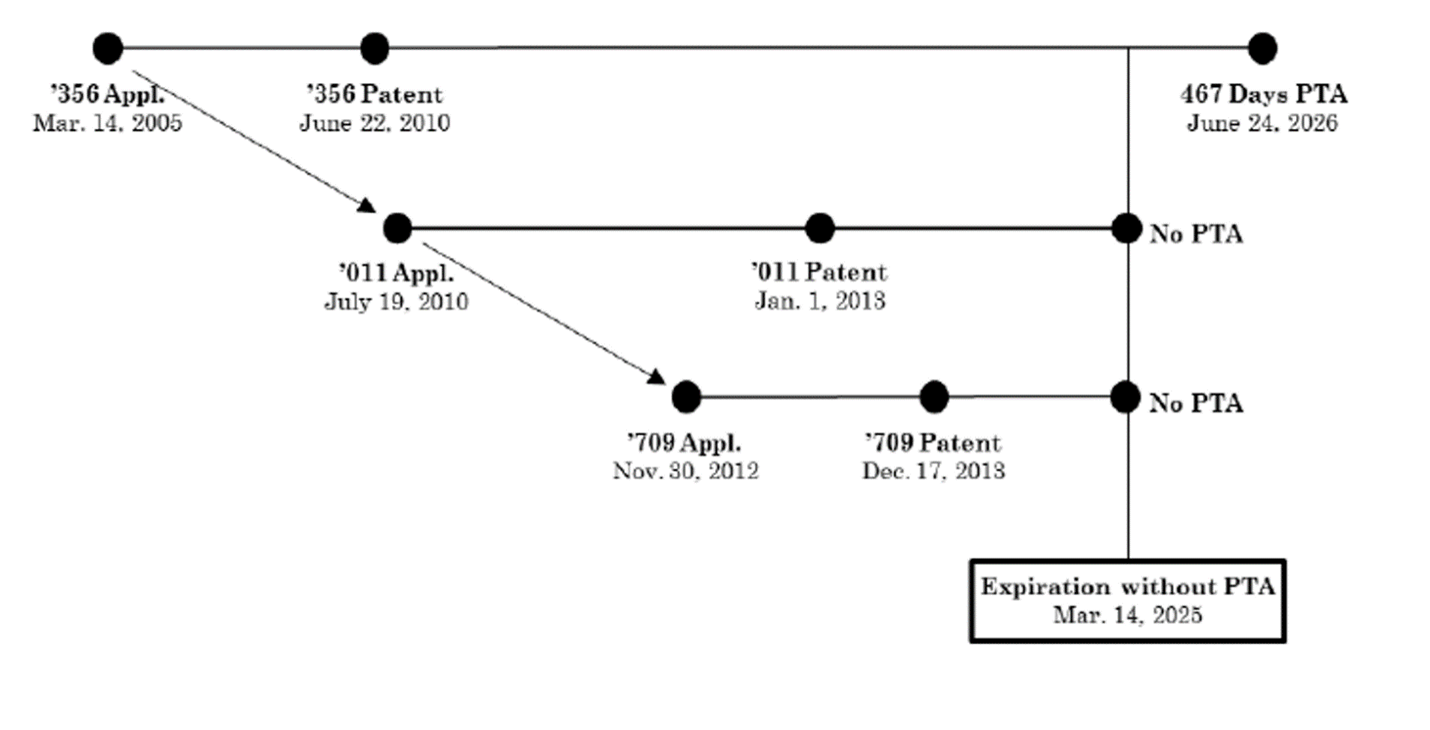
The challenged patent claimed a genus of eight compounds including eluxadoline, whereas the child reference patents claimed eluxadoline itself and methods of treatment using eluxadoline. The generics argued that the genus claimed in the challenged patent was an obvious variant of the claims to eluxadoline itself in the child reference patents. Relying on the court’s holding in Cellect, the district court held that the challenged patent was invalid for ODP over the child reference patents.
The Federal Circuit reversed, clarifying that although Cellect established a rule that courts must assess ODP based on expiration dates after PTA has been added, “[i]t does not follow […] that the [challenged patent] must be invalidated by the [child reference patents] simply because it expires later.” Rather, because both of the child reference patents were filed after, issued after and claimed priority to the challenged patent, neither could serve as an ODP reference to the challenged patent.
Central to the court’s reasoning was the underlying purpose of the ODP doctrine: “to prevent patentees from obtaining a second patent on a patentably indistinct invention to effectively extend the life of a first patent to that subject matter.” Here, the challenged patent was not a “second” patent, but in fact was the first patent in the patent family (whether judged based on filing or issue date), and it could not be invalidated over the later-filed, later-issued child patents.
Takeaways
This case establishes that the first-filed, first-issued patent in a family “sets the maximum period of exclusivity for the claimed subject matter and any patentably indistinct variants.” Patentees and practitioners should carefully consider the order in which various subject matter is pursued in a series of patent applications in the same family. The first-filed, first-issued patent in a family often receives the most PTA, and now cannot be invalidated for ODP over any subsequent patents in the same family. It often may be desirable to pursue the most critical subject matter (e.g., for a pharmaceutical patent application, species-level claims to a clinical product) in this first patent. The case reiterates the significant value of PTA, and why it is critical to consider the potential impact of any action that might reduce the amount of PTA awarded, such as taking an extension of time or filing a terminal disclaimer.
When analyzing a patent portfolio, practitioners should pay close attention to the filing and issue dates, in addition to expiration dates inclusive of PTA, for any families of US patents with similar claimed subject matter, in order to assess the risk of a double patenting challenge.
In litigation, such as Hatch-Waxman litigation, the Allergan decision will eliminate one potential defense for the first-filed, first-issued patent in a family. It will be increasingly important to work with experienced litigation counsel early in the patenting process to ensure that the first-filed, first-issued patent in a family has significant litigation value and to assess the need for terminal disclaimers in later family members.
[View source.]