
[author: Leslie Fischer*]
Recently, I had the privilege of speaking at the annual meeting of the American Society of Pharmacognosy in Colorado. Members of this scientific association are dedicated to identifying and isolating natural products from various sources, and finding use for these isolated compositions as medicines, cosmetics, food additives, etc. I don't imagine this group would normally be particularly excited to hear a patent attorney speak for an hour, but these are troubled times for natural products, and these folks were, indeed, troubled. Unfortunately, by the end of the evening, no one was feeling more positive about the patent eligibility of natural products in the United States. I don't relish going back next year with the news that the patent eligibility of claims reciting natural products has gotten even worse, which it has -- thanks to Ariosa Diagnostics, Inc. v. Sequenom, Inc. (Fed. Cir. 2015).
In subject matter eligibility (SME) cases, "natural products" traditionally fall within the judicial exception (JE) to 35 U.S.C. §101 referred to as a "natural phenomenon," the other two JEs being "abstract ideas" and "laws of nature." When we look to historical and modern court decisions addressing the eligibility of natural products, we find that product claims have been at issue, e.g., American Fruit Growers, Inc. v. Brogdex Co., 283 U.S. 1 (1931) (claims to oranges), Parke‐Davis & Co. v. H. K. Mulford Co., 189 F. 95, 103 (S.D.N.Y. 1911) (claims to adrenalin), Diamond v. Chakrabarty, 447 U.S. 303 (1980) (claims to genetically-modified bacteria), Association for Molecular Pathology v. Myriad Genetics, Inc., 569 U.S. __, 133 S. Ct. 2107 (2013) (claims to DNA), In Re Roslin, 750 F.3d 1333 (Fed. Cir. 2014) (claims to cloned sheep). The SME analysis used for product claims is quite different than the SME analysis used for process claims, and this makes sense -- natural products are tangible and concrete things, whereas abstract ideas and laws of nature are intangible concepts. For product claims reciting natural products, courts have asked (in one way or another) whether the composition or manufacture has some meaningful difference from its naturally‐occurring counterpart, whereas for process claims reciting abstract ideas or laws of nature, courts have asked (in one way or another) whether the idea or law recited therein is applied in a meaningful way. Ariosa is the first case that I am aware of in which an application test is used to analyze the SME of a process claim simply because it recites a natural product.
The process claims at issue in Ariosa relate to methods of detecting fetal DNA in maternal plasma, and, in some claims (e.g., claim 21 and 25), using that detected fetal DNA to provide a diagnosis. The Federal Circuit explains in the decision that the claimed processes begin and end with a natural phenomenon, and then nonchalantly concludes that the process claims are directed to naturally-occurring phenomenon (also called a "naturally‐occurring thing" by the panel). The panel is fairly clear that the alleged ending natural phenomenon is paternally‐inherited fetal DNA derived from maternal plasma (given the moniker "cell free fetal DNA" or "cffDNA" in the decision). But the panel is less clear as to what, exactly, is the beginning natural phenomenon, at one point identifying it as the existence of cffDNA in maternal blood ("It is undisputed that the existence of cffDNA in maternal blood is a natural phenomenon.") but in other passages suggesting that cffDNA itself is both the beginning and ending natural phenomenon ("Thus, the claims at issue, as informed by the specification, are generally directed to detecting the presence of a naturally occurring thing or a natural phenomenon, cffDNA in maternal plasma or serum. As we noted above, the claimed method begins and ends with a naturally occurring phenomenon.").
Having identified a natural phenomenon (or two)? in Sequenom's method claims, the panel was faced with a significant decision -- what relevant precedent to apply? cffDNA is, after all, a natural product, but historical and modern SME decisions related to natural products (e.g., American Fruit, Chakrabarty, Myriad, Roslin) analyze product claims, not process claims. And, historical and modern SME court decisions that analyze process claims (e.g., Parker v. Flook, 437 U.S. 584 (1978), Diamond v. Diehr, 450 U.S. 175 (1981), Mayo Collaborative Serv. v. Prometheus Labs., Inc., 566 U.S. __, 132 S. Ct. 1289 (2012), Alice Corp. Pty. Ltd. v. CLS Bank Int'l, 573 U.S.__, 134 S. Ct. 2347 (2014)) deal with very different JEs, i.e., abstract ideas and laws of nature. Each of these lines of cases is readily distinguishable. Undaunted, the Ariosa panel creates an entirely new rule of law, ostensibly derived from Mayo, but sounding rather like Flook. The Ariosa SME test may be broadly stated as follows: for process claims that encompass a natural product, the process steps themselves must be new and useful. While this SME test closely resembles Flook in that the process steps themselves must be inventive, it has a significant difference -- Flook's inventive application test has never been triggered by anything other than intangible JEs, i.e., abstract ideas and laws of nature. In contrast, Sequenom's claims recite only a tangible natural product.
Why is this extension of Flook problematic? First, the Flook (and Mayo) application‐based tests were designed to ensure that an intangible JE is meaningfully applied. Yet, many of Sequenom's claims, e.g., claim 1 and 24, are directed to methods of detecting, i.e., finding a substance, not using it. Hence, an application‐based test makes little sense for such claims. Second, the Ariosa panel has gone where no panel has gone before. In all three Federal Circuit Myriad decisions (Ass'n for Molecular Pathology v. USPTO, 653 F.3d 1329 (Fed. Cir. 2011), Ass'n for Molecular Pathology v. Myriad, 689 F.3d 1303 (Fed. Cir. 2012), University of Utah Res. v. Ambry Genetics Corp., 774 F.3d 755 (Fed. Cir. 2014)), the court addressed both product and process claims. For the product claims, which contained the "natural product" DNA, the precedents relied upon by the panels were product cases (i.e., Chakrabarty, Funk Bros. Seed Co. v. Kalo Inoculant Co., 333 U.S. 127, 130 (1948), American Fruit, Parke‐Davis, In re Marden, 47 F.2d 958 (CCPA 1931), Myriad, and Roslin). For the process claims, which also contained the "natural product" DNA, the precedents relied upon by the panels were process cases (i.e., Bilski v. Kappos, 561 U.S. 593 (2010), Diehr, Flook, Mayo, and Gottschalk v. Benson, 409 U.S. 63 (1972)). The SME analyses of process claims in the Federal Circuit Myriad decisions were not triggered by the presence of the "natural product" DNA in the claims, but rather by the presence of an abstract idea (i.e., the act of "comparing"). Only Ariosa takes the position that process claims encompassing a natural product trigger a SME analysis. In the SME matrix shown below, Ariosa creates a new law, and makes a new space.
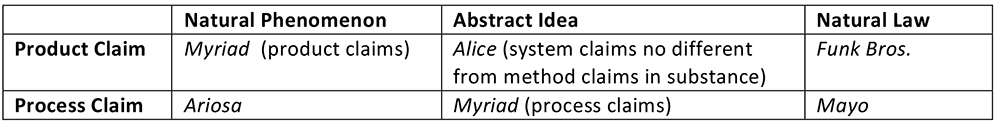
Rather than creating a new law for claims reciting natural products, the Ariosa panel could have looked to guidance from several Supreme Court decisions. In Mayo, Justice Breyer noted that a new way of using an old drug is an application of a law (read: patent eligible). But, in fairness, using fetal DNA for diagnostic purposes is not a new use of this substance, even though Sequenom's fetal DNA is clearly derived from a new source. Better is Judge Thomas's tantalizing Myriad passage that the claims in front of the court did not involve new applications of knowledge about the BRCA1 and BRCA2 genes, intimating, of course, that these types of claims are eligible for patenting. If the beginning "natural phenomenon" identified by the Ariosa court is the presence of fetal DNA in maternal serum, then surely an application of that knowledge is to assay maternal plasma for fetal DNA and use that information to provide a diagnosis (see, e.g., Sequenom's claim 21). The whereabouts of a molecule is not a concrete and tangible thing, but rather an observation, i.e., an intangible concept, far more like a law of nature (and in fact, is probably better described as a law of nature), and thus a JE amenable to an application-type SME test. Had the Ariosa panel clearly articulated the intangible nature of the JE it identified in the claims, then tangible natural products would be left out of this murky modern SME case (of course, one has to wonder about the soundness of any system in which the sheer existence of a substance in a particular location is a JE rendering suspect the eligibility of a claim).
Instead of taking advantage of Justice Thomas's offer, the Ariosa court created a chimera, i.e., a Flook-derived application‐based SME test applied to process claims reciting a natural product. Practitioners should be deeply concerned about how future panels (and the U.S. Patent Office) will apply this new animal. In the USPTO's December 2014 Guidance, process claims reciting only natural products, and no other JE, are not subjected to SME analysis, except in cases where a process claim is no different in substance from a product claim, e.g., "A method comprising providing a pomelo fruit." But, is this out of sync with Ariosa? In fact, claims many thought were safe following Mayo, e.g., a method of treatment (with or without a diagnostic aspect) using a natural product, may now fall prey to significant SME scrutiny. Imagine the claim: "A method of treating Disease D by administering Compound C to a patient having Biomarker B." Under a broad reading of Ariosa, the claim "encompasses" a natural product (Biomarker B). Using Ariosa's newly-minted Flook chimera, we ignore Biomarker B and ask whether the remaining aspects of the claim are unconventional or inventive. Assuming Compound C is old, Disease D is old, and methods for administering Compound C are old, what unconventional aspect remains? Why, the natural law that patients having Disease D and Biomarker B respond better to Compound C! But, natural laws are also ignorable JEs, and if we ignore that JE too, nothing remains in the claim that is "inventive." Imagine an even simpler claim: "A method of treating Disease D by administering Compound C to a patient." If Compound C is a natural product, then under a broad reading of Ariosa, the claim "encompasses" a natural product. Using the Flook chimera, we ignore Compound C and ask whether the remaining aspects of the claim are unconventional or inventive. Assuming Disease D is old and methods for administering Compound C are old, what unconventional aspect remains? Why, the natural law that patients with Disease D respond to Compound C! And if we ignore that JE, all that remains in the claim are old, well-known elements.
Why stop there? Let us take it a step further, extending Ariosa to product claims. After all, our courts seem dedicated to shoehorning all claim categories and all JEs into the same SME framework. Imagine an inventor is the first to discover that trypsin is absorbed by the ileum and can be administered orally to patients if enterically coated. The claimed enterically-coated trypsin molecule, analyzed under a broad extension of Ariosa, might not fare very well. Using the Flook chimera test, we ignore trypsin (a natural product) and ask whether the remaining claim elements are unconventional. Since enteric coating is conventional, the only unconventional aspect remaining in the claim is the natural law that trypsin can be absorbed by the ileum. Once we ignore that JE, the claim is empty of anything out of the ordinary. Far-fetched? These are essentially the facts of Armour Pharm. Co. v. Richardson Merrell, Inc., 396 F.2d 70 (3d Cir. 1968). In that case, the 3rd Circuit identified a natural law hidden within a product claim to enterically-coated trypsin (i.e., the natural law that the ileum absorbs trypsin). Citing Funk Bros., the 3rd Circuit pretended that the natural law was known in the prior art, and once done, the solution, i.e., enterically coating trypsin, was entirely conventional. While our §101 jurisprudence has, thankfully, gravitated away from these Funk-based shenanigans, Ariosa threatens to move us back to exactly that point for natural products.
How do we halt this devolution in our §101 jurisprudence?
First, our justices need to clearly identify the exact JE identified in each particular case, and into which category that JE falls (law of nature, abstract idea, or natural phenomenon). Should the JE be a "natural phenomenon", then further distinction is needed between concrete natural products and intangible observations. It is an important distinction whether the JE in Ariosa is the sheer existence of fetal DNA in maternal plasma (intangible law of nature or observation), or whether it is fetal DNA itself (tangible composition of matter), because U.S. courts address the SME of tangible versus intangible JEs differently.
Second, to select the proper SME test based on the proper precedent, a court must also be cognizant of the category of claim under scrutiny, i.e., product vs. process claims. If the JE identified in Sequenom's claims is the sheer existence of fetal DNA in maternal plasma, then the Ariosa court has identified an intangible JE, and, for process claims reciting an intangible JE, an application-based analysis could be appropriate. However, if the JE identified in Sequenom's claims is fetal DNA itself, the Ariosa court has identified a tangible molecule, and because the claims are process claims, no SME analysis is required (excepting a process claim that is no different in substance from a product claim). Cognizance and clarity as to both the type of JE and the particular claim category before a court is critical, as it allows selection and application of the correct legal precedent.
Third, and finally, we must resist the sirens' song to use the same SME test for every claim. In lieu of a single standard for analyzing the eligibility of all claims, our courts have set forth various different ways to satisfy §101. While the two-step framework of Mayo provides a guiding principle, the second step of Mayo, i.e., "what else is there in the claims before us?" does not dictate a process claim‐derived application‐based test for each and every claim. Alice, in fact, tells us that step two of the Mayo analysis is simply the search for an "inventive concept," i.e., an element or combination of elements amounting to significantly more than a claim to the JE itself. Mayo step two encompasses all the many ways the §101 inquiry has been historically satisfied, some precedent being applicable to product claims, some to process claims, some for tangible products, and some for intangible laws and ideas. But, there has never been one test that rules them all. And the more we try to fit everything into one neat little box, the more our §101 jurisprudence devolves, the more confused the Patent Office, practitioners and the public become, and the less likely it is that your valuable invention involving a natural product will see the light of day.
• Dr. Leslie Fischer, Ph.D., J.D., is an Adjunct Professor of Law at Seton Hall University