By Canadian Patent Utility Coalition* --
 While the United States and Canada share a border, common values and a strong commitment to international trade and security issues, many are surprised to learn that protection of intellectual property (IP) is a source of significant friction in our relationship. Indeed, in its 2014 annual "Special 301" report released last week on IP practices overseas, the Office of the United States Trade Representative (USTR) again placed Canada on its "Watch List" for inadequate IP protection and enforcement. In making this determination, USTR highlighted in particular that Canada's application of heightened patent utility requirements is "leading to uncertainty for patent holders and applicants and undermining incentives for investment in the pharmaceutical sector". In comments filed with USTR leading up to the issuance of its 301 report, a number of organizations -- including the U.S. Chamber of Commerce's Global IP Center (GIPC), Intellectual Property Owners Association (IPO), and the Canadian Patent Utility Coalition (CPUC) -- detailed the negative impact of Canada's heightened and improper patent utility standard on U.S. jobs, innovation and competitiveness. USTR's Special 301 process shines a spotlight on the degree to which the Canadian utility standard is contrary to internationally accepted norms and its own trade obligations.
While the United States and Canada share a border, common values and a strong commitment to international trade and security issues, many are surprised to learn that protection of intellectual property (IP) is a source of significant friction in our relationship. Indeed, in its 2014 annual "Special 301" report released last week on IP practices overseas, the Office of the United States Trade Representative (USTR) again placed Canada on its "Watch List" for inadequate IP protection and enforcement. In making this determination, USTR highlighted in particular that Canada's application of heightened patent utility requirements is "leading to uncertainty for patent holders and applicants and undermining incentives for investment in the pharmaceutical sector". In comments filed with USTR leading up to the issuance of its 301 report, a number of organizations -- including the U.S. Chamber of Commerce's Global IP Center (GIPC), Intellectual Property Owners Association (IPO), and the Canadian Patent Utility Coalition (CPUC) -- detailed the negative impact of Canada's heightened and improper patent utility standard on U.S. jobs, innovation and competitiveness. USTR's Special 301 process shines a spotlight on the degree to which the Canadian utility standard is contrary to internationally accepted norms and its own trade obligations.
The Issue
It is common for patents involving innovative medicines to be litigated. Competitors often argue that a patent should not have been granted because the invention was obvious or was not really new. However, it is extremely rare for patents to be challenged for lack of usefulness or utility. This is because patents are typically challenged by those who wish to revoke the patent and market the product themselves. If an invention is not useful, it makes little business sense to bear the costs of challenging the patent. Conversely, if the product is actually in production and being used by customers, it should be difficult to demonstrate that the invention is not useful and never should have been patented.
 Unfortunately, a pattern has developed in Canada whereby patents for innovative medicines are being challenged on the grounds that the inventions are not useful. Ironically, these challenges have come from companies seeking to have the patents revoked so that they can copy and market these medicines themselves. As a result, Canada has revoked valuable patents for nearly 20 useful medicines over the past nine years because the patents fail to satisfy Canada's unique patent standards. As noted by USTR, under this standard, courts are invalidating patents held by U.S. pharmaceutical companies on the basis of inutility, "even though such products have been in the market and benefiting patients for years".
Unfortunately, a pattern has developed in Canada whereby patents for innovative medicines are being challenged on the grounds that the inventions are not useful. Ironically, these challenges have come from companies seeking to have the patents revoked so that they can copy and market these medicines themselves. As a result, Canada has revoked valuable patents for nearly 20 useful medicines over the past nine years because the patents fail to satisfy Canada's unique patent standards. As noted by USTR, under this standard, courts are invalidating patents held by U.S. pharmaceutical companies on the basis of inutility, "even though such products have been in the market and benefiting patients for years".
Background: International and U.S. Patent Standards
Robust intellectual property protections are vital to the biopharmaceutical sector; adequate and effective patent protection, in particular, is what drives innovators to undertake enormous risks by investing in the research, development, and delivery of innovative new therapeutics to patients globally. The WTO Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS) and North American Free Trade Agreement (NAFTA) require patents to be granted for inventions relating to all fields of technology that are new, result from an inventive step, and are "capable of industrial application." "Capable of industrial application" is synonymous with "useful" and is often referred to as the "utility" standard. Thus, TRIPS and NAFTA require signatories to provide patent protection to useful inventions that meet the other requirements for patentability.
The utility standard is by no means intended to be a burdensome requirement. It is designed simply to ensure that patents are not granted for inoperable, fanciful, or purely aesthetic inventions. For example, in applying the patent utility test, the United States Patent and Trademark Office and U.S. courts require simply that an invention's claimed utility be specific and practical. The patent utility test applied in the U.S. is very similar to the tests applied in the European Union and Japan, among most other industrialized nations. When a patent is challenged for a lack of utility under Section 101 of the Patent Act, U.S. courts focus on actual utility and will consider evidence developed and submitted after the filing of the patent application in evaluating an invention's utility. Specific utility can be shown where a pharmaceutical or biotechnology patent discloses a specific disease against which the claimed compounds are useful.
Importantly, U.S. courts have made it abundantly clear that the human testing necessary for U.S. Food and Drug Administration (FDA) approval is not a prerequisite for finding usefulness for a therapeutic under the patent laws. The FDA's focus on human testing to demonstrate the safety and efficacy of a therapeutic before it is sold on the market is distinct from the Patent Act's patentability requirements. At the same time, USPTO and the U.S. courts accept evidence of FDA approval of human clinical trials as creating a strong presumption that the utility standard has been met. The U.S. approach is consistent with the practices of most if not all other WTO members. Canada's approach is the exception, and is inconsistent with international standards.
Canada's Patent Utility Standard: An Insolvable Riddle for Patentees
Canada's utility test has three elements which taken together present a riddle that is impossible to solve.
• First, the court or CIPO subjectively construes the "promise of the patent" from the patent specification, sometimes going beyond a mere statement of use and also beyond that which is specifically claimed in the patent application. There is no similar concept under U.S. law or practice. For example, "promises" have been construed as treatment of disease over the long term for conditions deemed chronic. There is no way to know in advance how the "promise" might be construed by the Court or Canada's Intellectual Property Office (CIPO).
• Second, the court or CIPO, using a heightened evidentiary standard of proof, determines whether utility is demonstrated by reference to the promise of the patent. As in the first test, there is no way a patentee can know in advance whether enough proof has been provided. For example, even completed human clinical trials have been found inadequate to demonstrate utility in Canada where the courts have questioned the size or duration of the trials. This stands in sharp contrast to U.S. practice, as noted above, where data from human clinical trials (even those trials conducted after filing of the patent application) create a presumption of usefulness.
• Third, if utility is not demonstrated, the court or CIPO determines whether, at the time of the filing, there was a "sound prediction of utility." In Canada, evidence of utility generated after the filing date is not considered and only evidence contained within the application may be used as proof of utility. This last prong creates heightened disclosure requirements inconsistent with U.S. practice, which does not require evidence of utility to be found within the specification as of the filing date.
The Consequences of Canada's Application of its Outlier Patent Utility Standard
Since the "promise" of the patent is construed by the court years after the filing date, the promise doctrine leads to great uncertainty among innovators as it is now unclear how much information is required at the time of filing to meet these new, onerous requirements. Moreover, this judicially-created utility doctrine applies a shifting standard that places applicants and patentees in an untenable "Catch-22" predicament: To be patentable, useful inventions must also be novel and inventive over all prior art available at the patent filing date. If an applicant aims to meet Canada's enhanced test for proof of utility, which may include carrying out long-term clinical trials prior to filing a patent application, the applicant would have to delay patent filings in Canada and other countries such as the United States. Such delays would increase the risk of patent refusal and patent invalidity in numerous countries on the basis of prior art published during the long-term clinical trials.
Canada's approach to utility is also inconsistent with the patent laws of similarly-situated economies and impedes ongoing efforts to achieve patent harmonization internationally. The Canadian approach is strikingly out of step with the first-to-file rule of the America Invents Act, which encourages early filing. It also compromises the ability of innovators to file patents in Canada using an international application under the Patent Cooperation Treaty (PCT), which has less onerous disclosure obligations. Moreover, the results in Canadian cases contradict those in the United States and Europe: pharmaceutical patents found to lack utility by Canadian courts have been upheld as having utility in U.S. and European proceedings, if utility is challenged at all.
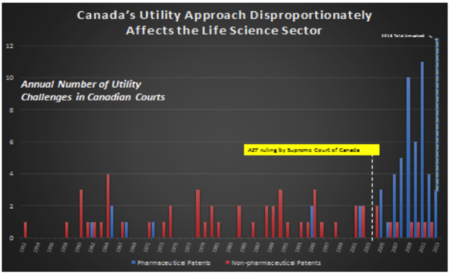
Moreover, Canada's promise doctrine, in practice, has discriminated against a particular area of technology -- the biopharmaceutical sector. Since 2005, all patent revocations based on utility in Canada have involved pharmaceutical patents.[1] Given the disproportionate impact of the promise doctrine on the biopharmaceutical sector, Canada is failing to meet its international obligation to apply patent standards in a non-discriminatory manner across different technologies. The graphic below illustrates the disproportionate impact on drug patents.
Conclusion
The heightened test applied by Canada is substantially different from the test required under TRIPS and NAFTA. Parties to these trade agreements cannot redefine core terms like "useful" and "capable of industrial application" without fundamentally changing the essence of the agreements. Canada's substantial redefinition of "usefulness" has severely undermined patent protection for innovators.
* The Canadian Patent Utility Coalition (CPUC) represents 19 innovative companies who are extremely concerned about Canada's heightened and improper patent utility standards which are causing significant economic harm and uncertainty to innovative companies.
[1] In only one case outside the pharmaceutical sector have any challenged claims been found to lack utility; a distinct claim under the same patent was upheld as useful, such that the patent remained valid. See Bell Helicopter Textron Canada Limitée v. Eurocopter, 2013 FCA 219.