All of us have probably had the experience of browsing the aisles at the grocery store looking for healthy foods to take home for our families. A few foods we find may include the word “healthy” on the packaging. Did you know that the FDA has actually defined what “healthy” on a food label means? Or that the FDA recently issued a new rule redefining the word “healthy”? While the meaning of the word “healthy” may seem simple, it is actually much more complicated for FDA labeling purposes than you probably imagined.
The FDA first promulgated a definition of “healthy” in 1994 and set standards that foods had to meet in order to include the word “healthy” on their labels. A new definition was finalized on December 19, 2024, with an effective date of April 28, 2025. Under FDA regulations, foods must comply with the new requirements in order to include the word “healthy” or derivatives of healthy like “healthful,” “healthfully,” “healthfulness,” “healthier,” “healthiest,” “healthily,” and “healthiness” on their labels.
So – what made the cut as “healthy”? As many would probably anticipate, vegetables and fruits, whether fresh, frozen, or canned (with only water) all made the cut as healthy. Some, perhaps, surprising foods which did not previously meet the FDA definition of “healthy” but now do, include olive oil, eggs, and salmon. While deciding what is “healthy” may seem simple, the new definition of “healthy” is rather complicated. In order to meet the new definition of “healthy” a food must:
- contain a certain amount of food from at least one of the food groups or subgroups (such as fruit, vegetables, grains, fat-free and low-fat dairy and protein foods) recommended by the Dietary Guidelines for Americans, and
- meet specific limits for added sugars, saturated fat and sodium.
A couple of acronyms important to understand the new definition of “healthy” are FGE and RACC. FGE stands for “food group equivalent,” and RACC means “Reference Amount Customarily Consumed.” The FDA rule defines an FGE as:
- Vegetable — 1/2 cup equivalent (c-eq)
- Fruit — 1/2 cup equivalent
- Grains — 3/4 ounce (oz) equivalent whole grain
- Dairy — 2/3 cup equivalent
- Protein foods:
- Game meat — 1 1/2 oz equivalent
- Seafood — 1 oz equivalent
- Egg — 1 oz equivalent
- Beans, peas, or lentils — 1 oz equivalent
- Nuts and seeds, or soy products — 1 oz equivalent
The FDA recognizes that for some foods the amount commonly consumed at a sitting – the RACC – may be smaller than the FGE.
Before diving into the logistics of FGEs and RACCs, let’s start with what is exempt from the new rule:
- Individual or mixed food products (which contain nothing other than the food product(s) and water), which are vegetables, fruit, whole grains, fat free and low fat dairy, lean meat, seafood, eggs, peas, lentils, nuts and seeds; and
- Water, tea, and coffee with less than 5 calories per RACC.
These products may be labeled as “healthy” because they are considered nutrient-dense foods.
Now for the more complicated calculations. Foods are divided into five categories: (1) an individual food with a RACC greater than 50 g or 3 tablespoons, (2) an individual food with a RACC less than 50 g or 3 tablespoons, (3) mixed foods, (4) main dishes, and (5) meals.
A food with a RACC over 50 g or 3 tablespoons can be labeled "healthy" only if it meets the FDA's requirements listed below. The same criteria apply to an individual food with a RACC of less than 50 g or 3 tablespoons, but the criteria are judged based upon a serving size of 50 g.

DV stands for daily value.
For mixed foods [21 CFR § 101.65(d)(3)(iii)] – meaning foods composed of elements from more than one food group – to be labeled as “healthy,” they must meet the following criteria per RACC.

A main dish [21 CFR § 101.13(m)] is one that substantially contributes to a meal and is defined as a dish:
- Weighing at least 6 oz per labeled serving; and
- Containing not less than 40 g of food, or combinations of foods, from each of at least two of the following four food groups . . .
- Bread, cereal, rice, and pasta group;
- Fruits and vegetables group;
- Milk, yogurt, and cheese group;
- Meat, poultry, fish, dry beans, eggs, and nuts groups; except that:
- None of the foods used to meet the criteria for a main dish can be sauces (unless they are in the sauces), gravies, condiments, relishes, pickles, olives, jams, jellies, syrups, breadings, or garnishes.
In order for a main dish to be able to be labeled “healthy” it must meet the criteria below:

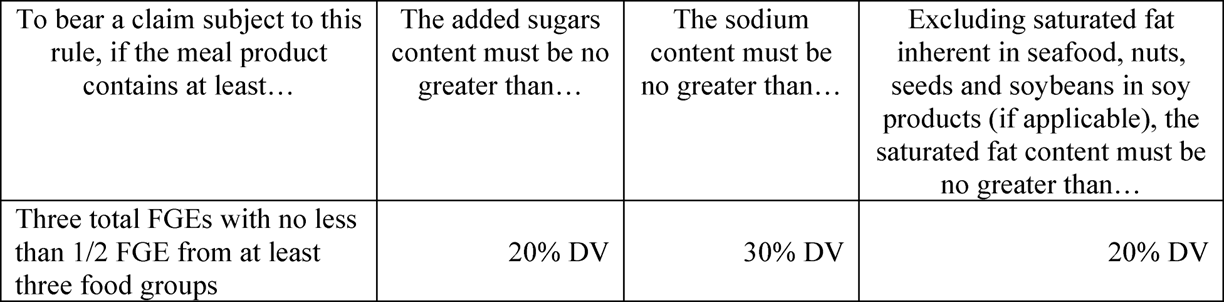
A meal [21 CFR § 101.13(l)], the fifth category, is defined as making a major contribution to an individual’s total diet and:
Weighs at least 10 ounces per labeled serving; and
- Contains not less than three 40 g portions of food, or combinations of foods, from two or more of the following four food groups:
- Bread, cereal, rice, and pasta group;
- Fruits and vegetables group;
- Milk, yogurt, and cheese group;
- Meat, poultry, fish, dry beans, eggs, and nuts group.
As with main dishes, none of the foods used to meet the criteria for a meal can be sauces (unless they are in the sauces), gravies, condiments, relishes, pickles, olives, jams, jellies, syrups, breadings, or garnishes. In order for a meal to bear the label of “healthy,” it must meet the following criteria:
The FDA requires comprehensive record keeping to substantiate all "healthy" claims. Manufacturers must provide evidence that their products labeled as "healthy" comply with the specified criteria during inspections. The exception to this is single ingredient foods which meet the definition of healthy. In the case of those foods, the ingredient list is the documentation. For other foods, thorough documentation must be provided to support the "healthy" claim.
Labeling food as "healthy" is voluntary, and most foods don’t claim it. However, manufacturers who choose to assert this claim must adhere to the regulatory definition of "healthy" to remain FDA compliant.
[View source.]