
In Novartis AG v. Ezra Ventures LLC, the Federal Circuit addressed a narrow but important question regarding its jurisprudence on the issue of obviousness-type double patenting (OTPD). That question was whether its decision in Gilead Sciences Inc. v. Natco Pharma Ltd., which established that a first patent filed earlier than a second patent but that issued later, could be used to invalidate the second patent on OTDP grounds, if the reason the later-expiring patent was later-expiring was due to Patent Term Extension under 35 U.S.C. § 156.
To recap, the Gilead Court decided that a later-issued but earlier-expiring patent could be used to establish OTDP over the later-expiring patent, as illustrated here:
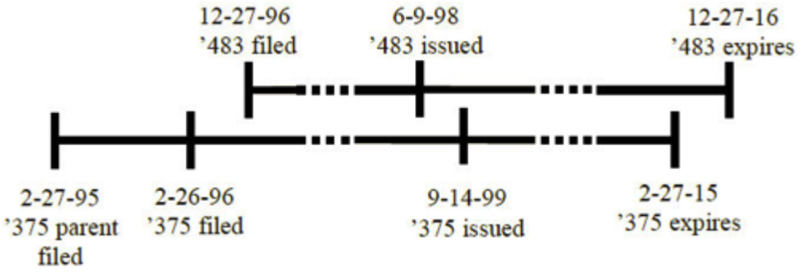
In rendering its decision, the Gilead panel held that the intervening change in U.S. patent term occasioned by ratification of the GATT-TRIPS agreement did not influence its decision. Rather, the panel grounded its decision on the "bedrock" principle that the public had a right to practice an invention (and its obvious variants) once a patent on that invention had expired. Important to the instant Novartis decision, the Gilead panel also voiced its concern that holding to the contrary in that case could raise the possibility of "significant gamesmanship" regarding patent term depending on when related applications were filed and when they were allowed to issue. The Gilead decision has been applied to mean that the relevant dates for considering OTDP are the expiration dates of the later and earlier-expiring patents, and whether the claims in the later-expiring patent were mere obvious variants of the earlier-expiring claims.
The Novartis case arose in ANDA litigation brought by Novartis against Ezra Ventures over its multiple sclerosis drug Gilenya® over Orange Book-listed U.S. Patent No. 5,604,229. As explained in the opinion, claims of the '229 patent encompassed fingolimod, the active ingredient in Gilenya®. The '229 patent was filed prior to the GATT-TRIPS changes in U.S. patent law, and thus had a patent term calculated as 17 years after issuance (in this case, corresponding to February 18, 2014). In addition, Novartis had been granted five years of Patent Term Extension under § 156, thus expiring on February 18, 2019.
Novartis owned another patent, U.S. Patent No. 6,004,565 that claims methods for administering fingolimod; this patent was filed after the U.S. changed how patent term was calculated under the GATT-TRIPS agreement and thus expired September 23, 2017 (20 years from its filing date). The filing and expiration dates of the '229 and '565 patents are related as illustrated below:
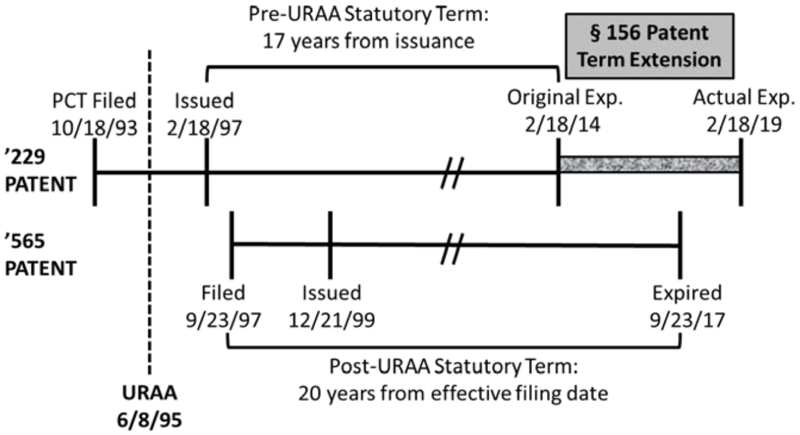
Before the District Court, Ezra Ventures filed a motion to dismiss (that the Court denied) under Fed. R. Civ. Pro. 12(c) for judgment on the pleadings, on three grounds. First, that extension of the '229 patent "de facto also extends the life of the '565 patent, and thereby violates § 156(c)(4)'s requirement that only 'one patent be extended.'" Second, that this extension "violates the 'bedrock principle' that the public may practice an expired patent." And third, that the '565 patent "renders the '229 patent invalid for statutory- and obviousness-type double patenting because Novartis's '229 patent claims are not patentably distinct from its '565 patent claims." With regard to the argument that this is an impermissible extension in violation of § 156(c)(4), the District Court held that "de facto" extension as argued here was inconsistent with the meaning of the extension statute. In addition, the lower court relied on the Federal Circuit's decision in Merck & Co. v. Hi-Tech Pharmacal Co., 482 F.3d 1317 (Fed. Cir. 2007), that the term of a terminally disclaimed patent can be extended under § 156, which "de facto" extends the term of that patent over the term of the patent over which it has been terminally disclaimed. Thereafter, Ezra Ventures stipulated infringement and withdrew its defenses, and the District Court entered final judgment against Ezra Ventures, clearing the procedural path for this appeal.
The Federal Circuit affirmed, in an opinion by Judge Chen joined by Judges Moore and Hughes. The panel first addressed Ezra Venture's argument that extension of the '229 patent by Patent Term Extension also (improperly) extended the term of the '565 patent. As an initial matter, the opinion notes that "nothing in the statute restricts the patent owner's choice for patent term extension among those patents whose terms have been partially consumed by the regulatory review process," despite it not being uncommon for a patented drug to also be the subject of patents on "a product, a method of using that product, and/or a method of manufacturing the product." The panel agreed with the District Court that Congress did not choose language that would preclude extension of a patent on a drug product because it also "effectively" extends the patent, for example, on a method of using that drug product. Here, only one patent term was extended -- the '229 patent -- and this satisfies the statutory mandate and does not violate § 156(c)(4).
Next the Federal Circuit considered the "interaction" between § 156 and OTDP. In what the panel states is "a logical extension" of its holding in Merck & Co. v. Hi-Tech Pharmacal Co., the Court held here that OTDP does not invalidate extension of the patent term under § 156. After explicating the Court's basis for its Merck opinion (inter alia, the plain meaning of the § 156 and the differences in statutory language between § 156 and § 154, the patent term adjustment statute, which cannot extend the term of a terminally disclaimed patent) the opinion notes that the Merck decision involved just the issue raised here: that a terminally disclaimed patent can still receive the benefit of a patent term extension under § 156. Accordingly, extension of the term of the '229 patent past the expiration date of the '565 patent was not improper.
It is when the opinion turns to "Ezra's policy concerns" that the panel took the opportunity to set forth its reasoning on the question of the function of the OTDP doctrine. Citing Proctor & Gamble Co. v. Teva Pharm. USA, Inc., 566 F.3d 989, 999 (Fed. Cir. 2009), the opinion states that the instant situation "does not raise the traditional concern with obviousness-type double patenting of a patent owner 'extending his exclusive rights to an invention through claims in a later-filed patent that are not patentably distinct from claims in the earlier filed patent.'" The opinion then distinguishes the policy considerations at play here with more recent OTDP decisions of the Court, particularly Gilead Sciences, Inc. v. Natco Pharma Ltd., specifically referring to that Court's concerns with gamesmanship not present here.
The other distinction between the situation in Gilead and the one here is whether the differences in the relationship between the patents (patents filed pre- and post-URAA here, in contrast to having two post-URAA patents in Gilead) are addressed in the opinion accompanying this one, Novartis Pharmaceuticals Corp. v. Breckenridge Pharmaceutical. That case will be the subject of a later post.
Novartis AG v. Ezra Ventures LLC (Fed. Cir. 2018)
Panel: Circuit Judges Moore, Chen, and Hughes
Opinion by Circuit Judge Chen