
Pharmaceutical patent owners have been one of the more vocal groups decrying the creation and existence of inter partes reviews and other PTAB post-issuance proceedings. And for good reason. Congress enacted the Hatch-Waxman statute to create an abbreviated approval pathway for generic small molecule drugs. In so doing, it crafted a fine balance between the interests of branded pharmaceutical NDA holders and generic drug-product manufacturers. As part of this balance, Congress established a procedure by which ANDA filers could challenge the validity of patents that cover the drug product and methods of its use in district court without needing to launch at risk. This included the creation of the Orange Book, which listed all such patents. An ANDA filer wishing to challenge these patents files a Paragraph IV certification for each patent that it believes is not infringed, is invalid, or is unenforceable. Technically, however, it is the filing of the ANDA that is the act of infringement regardless of whether the patent is Orange Book listed or not. Nevertheless, with the introduction of IPRs and related proceedings, ANDA filers have a new mechanism to challenge the validity of these patents outside the carefully balanced patent-resolution scheme of the Hatch-Waxman statute. Correspondingly, the new PTAB proceedings have the potential to shift the balance away from patent and NDA holders and towards generic drug manufacturers.
Likely in response to these concerns, Chief Judge Ruschke announced the results of an Orange Book-listed Patent Study during his "Chat with the Chief" on March 13, 2018. The meeting presentation slides can be found here. During the chat, the Chief Judge also revealed the results of an expanded panel study, which we will analyze in a future post. For the Orange Book study, the PTAB classified a petition as challenging an Orange Book-listed patent if, unsurprisingly, the patent was listed in the Orange Book when the petition was filed. The data provided was through the end of the fiscal year 2017, which ended on September 31, 2017. We will look at three of the questions asked by the Office in this study.
What Are the Filing Trends for Petitions Challenging Orange Book-listed Patents?
The PTAB has been releasing filing trends for the various technology centers since at least April, 2015. For fiscal year 2015, Bio/Pharma patents from TC 1600 accounted for 9% of all petitions filed. For fiscal year 2017, that number was 11%. However, it is important to keep in mind that this is a relative number, and that the total number of Bio/Pharma petitions has risen every year (despite the relative number hovering around 10%). The current study broke down the numbers in this technology center by whether it was an Orange Book-listed patent or a miscellaneous Bio/Pharma patent. The results since September 16, 2012, and the results for fiscal year 2017 are reproduced below.
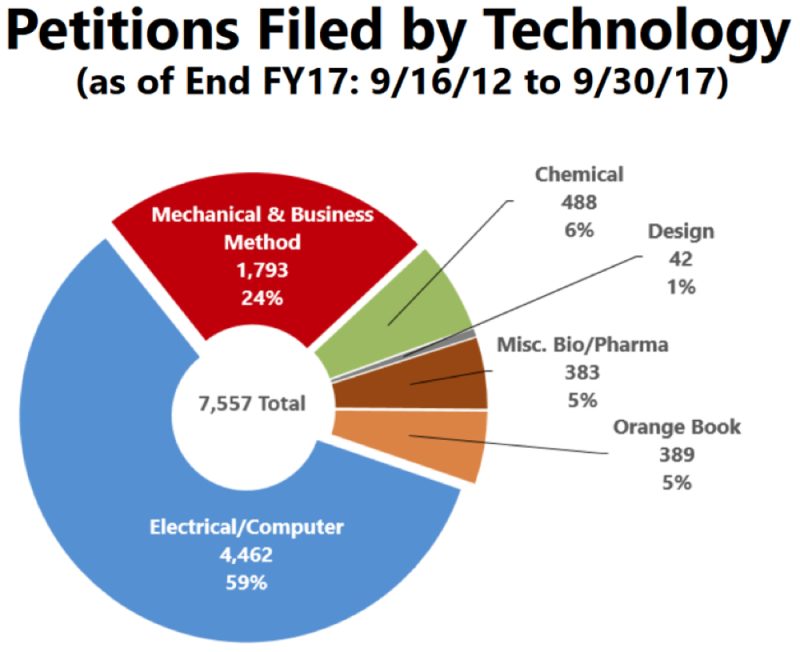
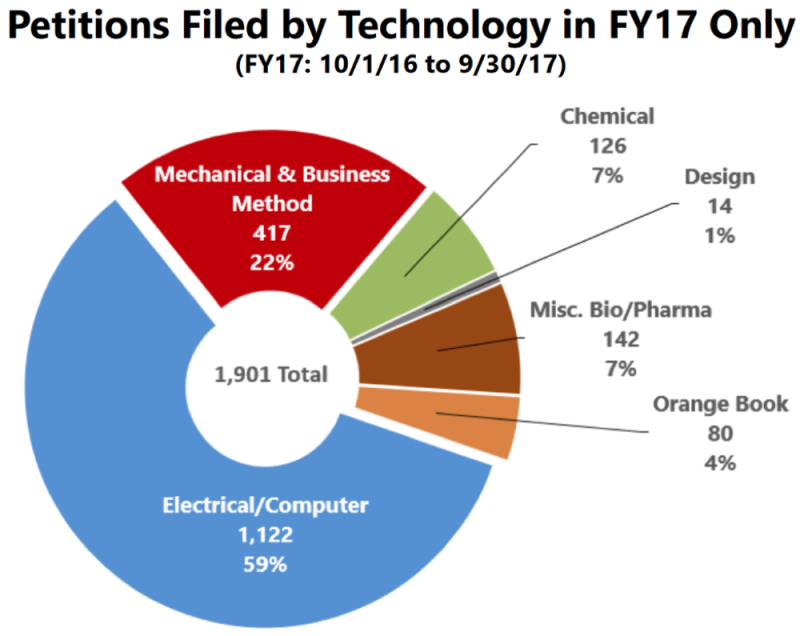
The picture that emerges is that, overall, Orange Book-listed patents have accounted for about half of all Bio/Pharma petitions, but that this ratio has recently shifted away from such patents. This could be due to the rise in the use of IPRs and other post-grant proceedings in the BPCIA context. The Patent Office also included the actual number of petitions that fell into the Orange Book-listed category, and it appears that such petitions peaked in FY15, with the number of petitions filed in FY17 being about 2/3 of the numbers filed in FY16 (80 versus 127).
How Does the Institution Rate on Petitions Challenging Orange Book-listed Patents Compare to Those of Other Technologies?
The Patent Office highlighted the fact that the cumulative institution rate of Orange Book petitions was essentially the same as the cumulative overall rate (66% versus 68%). Interestingly, the institution rate of the miscellaneous Bio/Pharma patents was 60%, lower than both. Moreover, whereas it would have made the study more complicated, it would have been interesting to break out the institution rate number by whether the patents contained composition-of-matter claims or method-of-use claims.
What Are the Outcomes of Challenges to Orange Book-listed Patents?
When looking at the 82 total final written decisions for Orange Book-listed patents, the Office found that more than 50% retained all claims as patentable. This is an extraordinary number, considering the number for all other technologies was 17%. The charts are reproduced below:
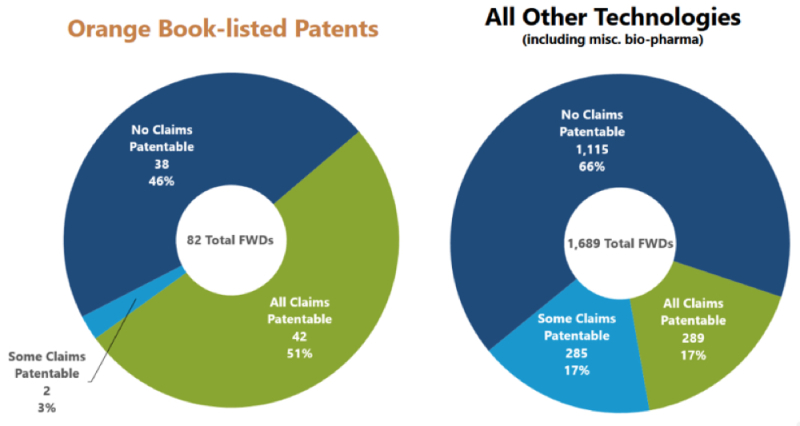
Nevertheless, the number one "number" highlighted by the study was that 83% of all such petitions resulted in the patent being unchanged. This is represented in the below reproduced graphic:
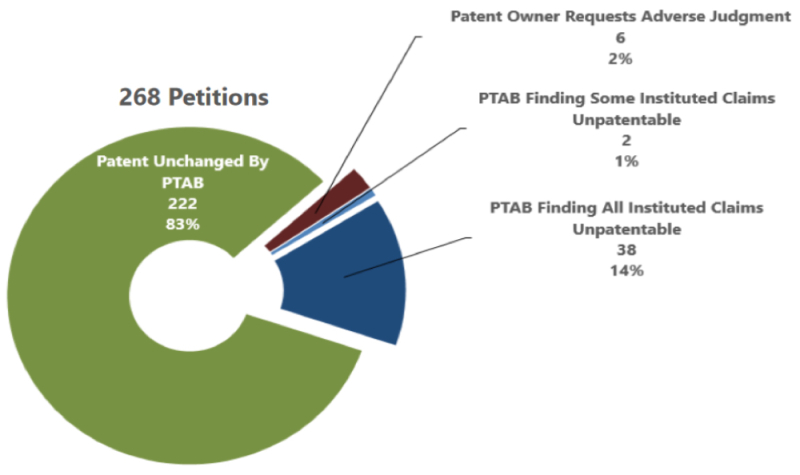
Touting this number seems suspect, however. The Patent Office considered a patent as being unchanged when it survived final written decision, when the parties settled, when the case was dismissed, and when the case was not instituted in the first place. In addition to the aforementioned success of such patents at the final written decision stage, there are unique factors intrinsic to Orange Book-listed patents that may help explain this number. For example, Orange Book-listed patent holders may be more motivated to settle with parties in order to retain the listing (and thereby prevent the flood-gates of ANDA filers from opening). In addition, many petitioners are not the first ANDA filers, and the NDA holder may have already settled with the first filer. In such cases, there may be less of a barrier to settle with follow-on challengers. Importantly, the patent holder in such situations may not consider a settlement a positive outcome.
The other two conclusions reached by the study relate to the issue of serial petitions. In general, the Office has been concerned about multiple petitioners filing against the same patent. However, the problem seems more pronounced in the Orange Book context because of the potential for multiple ANDA filers for a particular drug product. Nevertheless, the Office found that 80% of all challenged Orange Book-listed patents have only 1 or 2 petitions filed against it, as opposed to 87% for all challenged patents. Moreover, 85% of all challenged Orange Book-listed patents have 1 or 2 petitioners, as opposed to 94% for all challenged patents. These numbers are presented in such a way to suggest that the problem is not as significant as feared. However, what isn't represented is that 1/3 of all Orange Book-listed patents are challenged by more than one petitioner (as compared to 15.6% for all challenged patents). Moreover, according to this study, 1/3 of all patents are challenged by more than one petition, but for Orange Book-listed patents, the number is closer to 42%. Therefore, there does seem to be a multiple-petition or multiple-petitioner problem with this subset of patents.
In any event, this study by the Patent Office is certainly interesting. It does go a long way to allaying the fears of NDA holders. Nevertheless, considering that an IPR is a lose-or-draw proposition for any patent holder, and that Orange Book-listed patents are so valuable to NDA holders, this may come as little comfort to the community. Instead, anything that disrupts the balance struck by Hatch-Waxman is not going to be seen as favorable, no matter how the actual numbers have been borne out.