On October 5, 2023, the World Intellectual Property Organization published Cast21’s PCT application related to its alternative cast device – a 3D-printed exoskeleton created from a medical-grade resin. Cast21’s device seeks to address limitations in one of orthopedic care’s fundamental treatment methods: the cast. A soggy cast can lead to infections, reduced support, skin infections, and more. But as discussed in Cast21’s white paper, Cast321’s FDA-approved product touts a waterproof and breathable design, allowing patients to shower, swim, or wash their limbs. Cast21’s website provides a comparison with traditional casts:

A patent that issued in June 2022 explains that the Cast21 product includes a “lattice structure.” A “liquid resin and a catalyst mixture that transforms into a solid” is inserted into the lattice structure (see the patent’s Claim 1 and Fig. 1A).
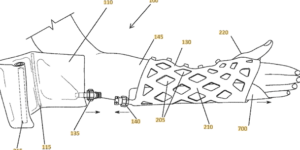
The patent application that published in October 2023 seeks additional patent coverage for related cast configurations and accessories.