
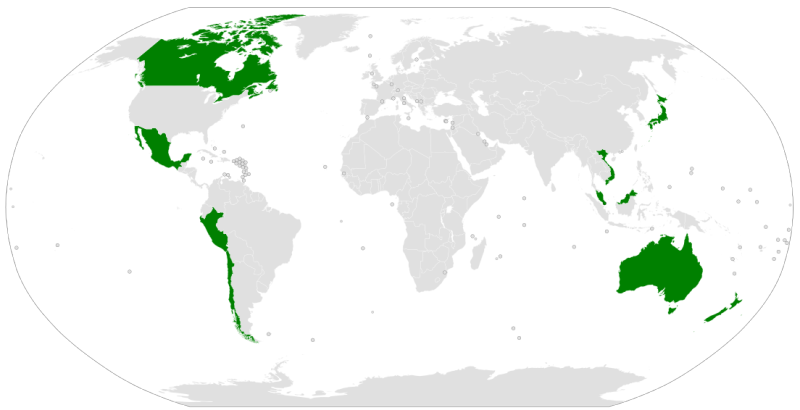
One of Donald J. Trump's signature campaign promises was to reject the Trans-Pacific Partnership negotiated by the Obama administration, and true to his word he did just that almost immediately upon being sworn into office (see "Why President Trump Is Wrong about Trans-Pacific Partnership Agreement"). This action raised questions about the fate of the agreement, which had as a trigger for coming into force that the signatories make up 34% of global GDP. Last July, representatives of the "TPP-11" (all of the TPP member states other than the United States, i.e., Australia, Brunei, Canada, Chile, Japan, Malaysia, Mexico, New Zealand, Peru, Singapore, and Vietnam) met to discuss implementation of the TPP without U.S. involvement. They indicated then that they would continue to meet and negotiate, and expressed hope that they would be able to finalize a trade agreement by the end of the year having only minimal differences from the original text of the TPP agreement.
Today, the Financial Times reported that the TPP-11 have indeed come to an agreement and plan on ratifying the TPP in March, sourcing the information from the Japanese government. The process has resulted in a name change: the agreement is now called the Comprehensive and Progressive Agreement for Trans-Pacific Partnership or CPTPP and, according to FT, "lays down a marker to China by setting high legal standards for trade and opens up the possibility of other Asian countries joining the pact."
As a reminder of opportunities lost, and provided there have been no wholesale changes in the treaty during current negotiations, the IP provisions of the treaty have broad scope, encompassing copyrights, trademarks, patents, and trade secrets. These provisions are aimed at establishing a minimum level of protection among the member states, and to harmonize such protections where possible. The patent provisions define eligible subject matter broadly, for "any invention, whether a product or process, in all fields of technology, provided that the invention is new, involves an inventive step and is capable of industrial application." Market exclusivity provisions regarding agricultural products are granted for at least 10 years, and regulated pharmaceutical products are entitled to at least five years of market exclusivity. Signatories will provide a legal framework for the pharmaceutical license holder to challenge approval and marketing of any generic version of a patented drug. Biologic drugs are afforded at least eight years of market exclusivity, or at least five years combined with other regulations in a signatory country that would result in at least eight years of exclusivity. All these provisions are subject to further review by the signatories after 10 years, to provide the ability to adapt the exclusivity term based on experience. These exclusivity terms—which are shorter than those available to biologic drug innovators in the United States (12 years) or Europe (10 years)—are longer than the terms (i.e., no exclusivity term) available in many of the signatory states. These provisions provided in signatory countries the prospect that patent protection would be supported by regulatory regimes that encourage investment in creating distribution and professional networks to bring patented pharmaceuticals to these populations that might otherwise be unattractive for such investment. Paradoxically in view of those that believed the treaty would preclude access to lifesaving medicines in developing countries, having this framework might actually have increased the likelihood that such drugs become more generally available in those countries.
The agreement also contains enforcement provisions for protecting IP rights, aimed at "permit[ting] effective action against any act of infringement of intellectual property rights covered by this Chapter, including expeditious remedies to prevent infringements and remedies that constitute a deterrent to future infringements," available in equal measure for patent, copyright, or trademark infringement. In addition to damages and the possibility of an injunction against future infringement, the agreement empowers signatories to destroy infringing articles, particularly counterfeit goods, and there are particular provisions relating to counterfeit articles identified at a signatory's borders. The TPP further provides, for the first time in an international trade agreement, criminal penalties for trade secret theft, as well as criminal enforcement provisions for willful trademark or copyright infringement including counterfeiting, intercepting or transmitting without authorization an encrypted program-carrying cable signal, and provisions relating to Internet service providers with regard to preventing unauthorized use of copyrighted materials.
The good news for U.S. biologic drug companies is that there are now opportunities for the benefits of the pact to accrue to them, provided that they partner with indigenous companies in member countries or resource their biologic drug production to an affiliate in a TPP country in order to reap the 8-year market exclusivity benefits under the treaty. Potential bad news includes the loss of U.S. leadership and cultivation of and protections for U.S. interests amongst the signatories. Potentially worse news is the potential for China to enter the vacuum of leadership created by U.S. withdrawal. China's participation is evidently contemplated by the TPP-11 (or perhaps more accurately, CPTPP-11), and such entry could further exacerbate trade imbalances that paradoxically were a prime motivation for the current administrations "America First" policy proclivities. While such sloganeering may create cheering when broadcast to the American electorate, global realities may be much less accommodating to the outcome desired by this Administration's supporters. One thing is clear: the CPTPP is unlikely to suffer the fate of other American initiatives abandoned by succeeding administrations (like the League of Nations), and we will likely not be happy when we are on the outside looking in.
Image of Membership of the Trans-Pacific Partnership by JayCoop, from the Wikimedia Commons under the Creative Commons Attribution-Share Alike 4.0 International license.