
Summary
Earlier this month, the United States Department of Agriculture (USDA), Food Safety and Inspection Service (FSIS) issued a proposed rule to overhaul the nutrition facts panel for meat and poultry products. FSIS issued its proposal in part to parallel the U.S. Food and Drug Administration’s (FDA or the Agency) final rule that the Agency issued earlier this year to revise the nutrition facts panel for packaged foods. The proposed rule, if finalized, would pose significant compliance challenges.
Among other things, the proposed rule would:
-
revise the format of the nutrition facts panel;
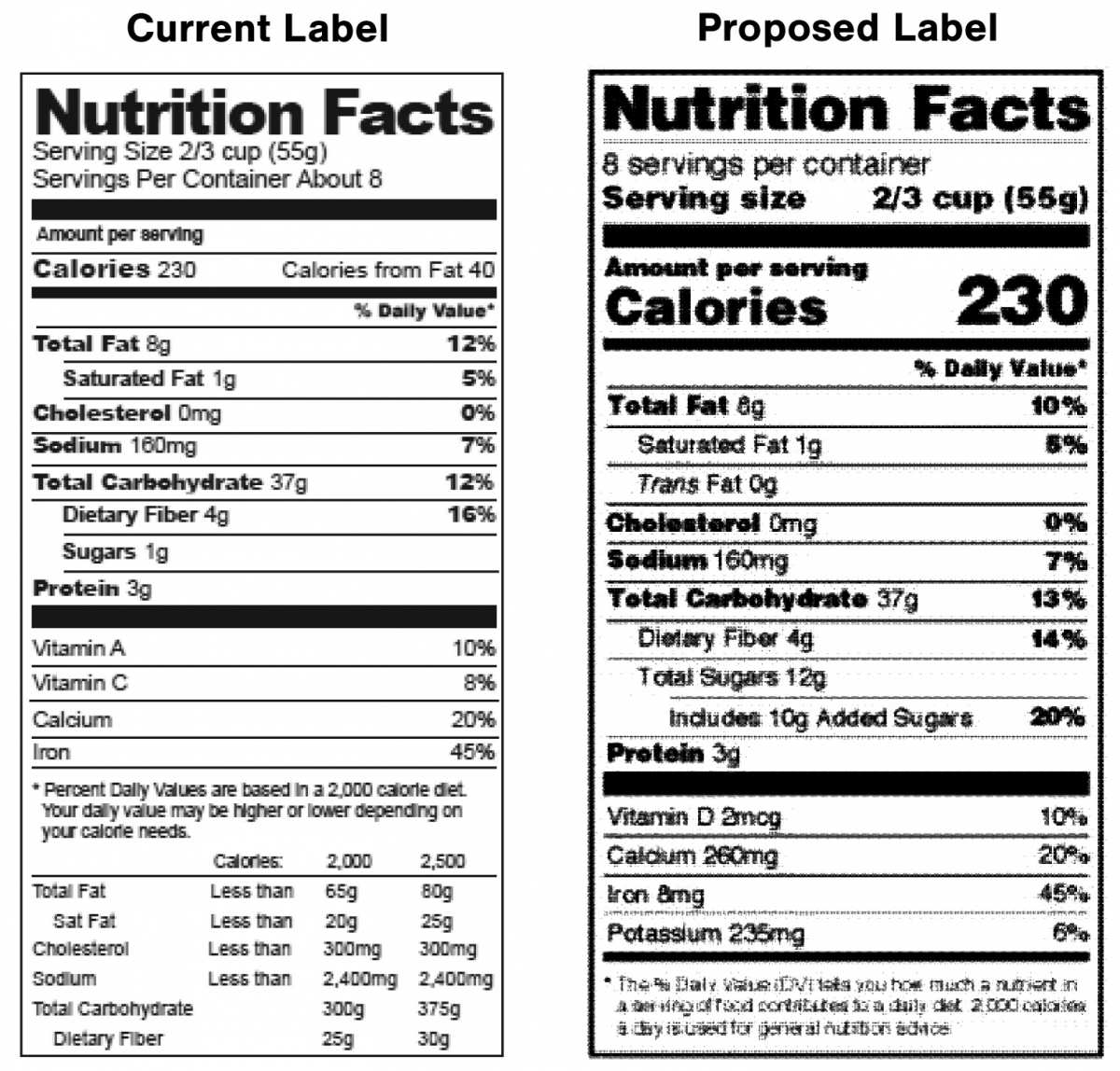
-
update the list of nutrients that are required or permitted to be declared on the nutrition facts panel;
-
update Daily Reference Values (DRVs) and Reference Daily Intake (RDI) values based on current dietary recommendations;
-
amend the labeling requirements for foods for children under the age of 4 years and pregnant women and lactating women, and establish nutrient reference values for these populations;
-
amend the definition of a single-serving container; and
-
update and modify certain reference amounts customarily consumed (RACCs).
Consistent with FDA’s regulation, USDA is proposing to remove certain requirements from the nutrition facts panel, such as “calories from fat,” while adding others, including required declarations of “added sugars,” Vitamin D, and potassium, and voluntary declarations of Vitamins A and C. Also, like FDA, USDA is proposing to update the reference value for the declaration of percent Daily Value (DV) for sodium from the current value of 2,400 milligrams (mg) to 2,300 mg.
As with FDA’s final rule, USDA’s proposed rule, if finalized, would pose significant compliance challenges. In addition to determining how much added sugars, Vitamin D, and potassium are present in their products, meat and poultry firms would also need to calculate the percentage DV based on the proposed rule’s updated DV values for certain nutrients. Beyond the percentage DV calculations, it is possible that meat and poultry products would need to remove nutrient content claims from their product labels or reformulate products in order to continue to make such claims. This results from the proposed rule’s changes in the RACC categories, changes in DV for certain vitamins and minerals, and modifications to the definition of fiber to exclude certain isolated and synthetic fibers.
Interestingly, and unlike FDA in its final rule, USDA does not explain in its proposed rule why required declaration of “added sugars” does not violate the First Amendment rights of covered firms. In contrast, FDA discussed compelled speech at length in its rule, and why, in the Agency’s opinion, FDA’s decision to compel food firms to disclose added sugars meets the three-part test set forth by the Supreme Court in Zauderer v. Office of Disciplinary Counsel of Supreme Court. 471 U.S. 626 (1985). Just as litigation is expected over FDA’s decision to compel declaration of “added sugars,” it is likely to expect that similar litigation would follow finalization of USDA’s rule.