
 In a forum held last month on the U.S. Patent and Trademark Office's Alexandria campus, Office representatives discussed the Interim Guidance on Patent Subject Matter Eligibility, which was released in December, and received additional input from the public regarding that guidance. The forum, which ran almost four and a half hours, began with some opening remarks by Drew Hirshfeld, Deputy Commissioner for Patent Examination Policy at the USPTO, and then Raul Tamayo, Senior Legal Advisor in the Office of Patent Legal Administration at the USPTO, followed Mr. Hirshfeld's presentation with an overview of the Interim Guidance. The remainder of the forum was devoted to two groups of public presentations -- in which the presenters were essentially divided into those on the life sciences side (group one) and those on the hi-tech side (group two) -- and an "open participation/question/answer" session, in which attendees were allowed to give statements or ask questions of USPTO representatives participating in the forum. A replay of the forum can be viewed here.
In a forum held last month on the U.S. Patent and Trademark Office's Alexandria campus, Office representatives discussed the Interim Guidance on Patent Subject Matter Eligibility, which was released in December, and received additional input from the public regarding that guidance. The forum, which ran almost four and a half hours, began with some opening remarks by Drew Hirshfeld, Deputy Commissioner for Patent Examination Policy at the USPTO, and then Raul Tamayo, Senior Legal Advisor in the Office of Patent Legal Administration at the USPTO, followed Mr. Hirshfeld's presentation with an overview of the Interim Guidance. The remainder of the forum was devoted to two groups of public presentations -- in which the presenters were essentially divided into those on the life sciences side (group one) and those on the hi-tech side (group two) -- and an "open participation/question/answer" session, in which attendees were allowed to give statements or ask questions of USPTO representatives participating in the forum. A replay of the forum can be viewed here.
Summaries of Mr. Hirshfeld's and Mr. Tamayo's presentations were provided in an earlier post. Today, we provide some of the highlights from the first group of public presentations, which consisted of nine presenters: Leslie Fischer of Novartis Pharmaceuticals Corp.; Hans Sauer of the Biotechnology Industry Organization (BIO); Courtenay Brinckerhoff of Foley & Lardner LLP; Suzannah Sundby of Canady + Lortz LLP; Anthony Sabatelli of Dilworth IP, LLC; Brian Stanton of Stanton Consulting Services, LLC; Hathaway Russell of the Coalition for 21st Century Medicine; and Amelia Baur and Kevin Greenleaf of the ABA Section of Intellectual Property.
During her presentation, Dr. Fischer argued that Step 2B of the Interim Guidance's test -- which corresponds to part 2 of the Alice Corp./Mayo test, and which calls for the examiner to "[d]etermine whether any element, or combination of elements, in the claim is sufficient to ensure that the claim amounts to significantly more than the judicial exception" -- may not be a proper interpretation of case law. Pointing out that "there is only one Rome, but there are many roads to Rome," Dr. Fischer contended that "one test doesn't rule them all" and asked the Office to consider redrafting Step 2B to encompass other tests. She suggested that one of those tests -- the markedly different analysis for nature-based products that the Office had placed in Step 2A -- should be moved to Step 2B. A copy of Dr. Fischer's presentation can be obtained here.
Dr. Sauer addressed the impact of the Interim Guidance on innovation, noting that while several media reports had recently described the discovery of the first new antibiotic in thirty years (see, e.g., "U.S. Scientists Discover Powerful New Antibiotic"), those reports had failed to mention that a patent application directed to the antibiotic had been rejected by the USPTO. (Interestingly, the announcement of the discovery of the new antibiotic came only months after the President's Council of Advisors on Science and Technology issued a report for dealing with the problem of antibiotic resistance.) Dr. Sauer indicated that the President had touched on antibiotics in his State of the Union address, and reminded the Office that it was charged by statute with informing the President about patent issues such as subject matter eligibility.
Ms. Brinckerhoff's presentation focused on the Federal Circuit's decision in In re BRCA1- and BRCA2-based Hereditary Cancer Test Patent Litigation ("Myriad II") which issued one day after the Interim Guidance was released (a decision that Dr. Sauer noted had tempered the optimism of the patent community for the Interim Guidance). According to Ms. Brinckerhoff, in Myriad II, the Federal Circuit ignored Supreme Court precedent and reached the wrong decision by confusing a molecule's property with its function (see slide below from her presentation).
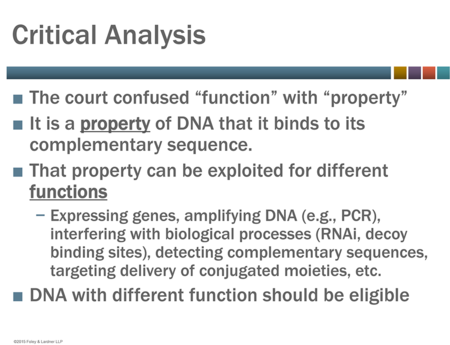
A copy of Ms. Brinckerhoff's presentation can be obtained here.
Ms. Sundby's presentation addressed the impact of the Interim Guidance on "weighted" assay claims (diagnostic method claims wherein weighted values are assigned to a number of biomarkers and a diagnosis is made based on a total weighted value for those biomarkers). She argued that the biomarker weights had been assigned by the inventor and were not simply the discovery of a law of nature, and therefore that such claims should be found to be patent eligible. A copy of Ms. Sundby's presentation can be obtained here.
Dr. Sabatelli discussed some of the positive aspects of the Interim Guidance (e.g., discarding the 12-factor test) and then provided a critique of certain aspects of the Guidance that required further improvement. Echoing Dr. Fischer's comments, much of Dr. Sabatelli's criticism focused on the "detour" for nature-based products (see slide below from his presentation).
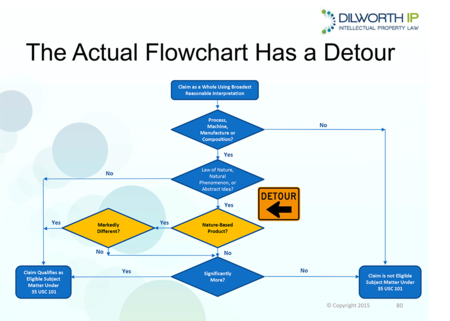
Dr. Sabatelli's proposed solution was to "ditch the detour." A copy of Dr. Sabatelli's presentation can be obtained here.
Mr. Stanton began his presentation by noting that he had worked on the written description guidelines during his tenure at the USPTO (he had since left the Office for private practice). With respect to the Interim Guidance, he provided a few proposals for improving the Guidance, including focusing on the issue of preemption in conducting a subject matter eligibility analysis, and establishing a working group that could propose claims which would serve as templates for drafters. He also called on the Office to release the long overdue report on genetic testing that was mandated by the Leahy-Smith America Invents Act.
Highlights from the second group of public presentations will be provided in a subsequent post.
For additional information regarding this topic, please see:
• "USPTO Holds Forum on Interim Guidance -- Part I," February 2, 2015
• "USPTO Issues Post-Alice Abstract Idea Examples," January 28, 2015
• "Impact of Interim Guidance on Business Method and Software Claims," December 17, 2014
• "USPTO Issues Interim Guidance on Subject Matter Eligibility," December 16, 2014
• "USPTO to Release Revised Subject Matter Eligibility Guidance," December 15, 2015