 Last Friday, the U.S. Patent and Trademark Office held a four-hour long forum to receive public feedback on the Myriad-Mayo Guidance, which was issued by the Office on March 4. According to the Office's Guidance webpage, the forum was intended to provide an opportunity for stakeholders to present their interpretation of the impact of Supreme Court precedent on the complex legal and technical issues involved in subject matter eligibility analyses during patent examination. On Monday, we reported on the opening remarks provided by Drew Hirshfeld, Deputy Commissioner for Patent Examination Policy, and the overview of the Guidance provided by Raul Tamayo, Senior Legal Advisor for the Office of Patent Legal Administration. Today, we report on the comments provided by several of the public presenters.
Last Friday, the U.S. Patent and Trademark Office held a four-hour long forum to receive public feedback on the Myriad-Mayo Guidance, which was issued by the Office on March 4. According to the Office's Guidance webpage, the forum was intended to provide an opportunity for stakeholders to present their interpretation of the impact of Supreme Court precedent on the complex legal and technical issues involved in subject matter eligibility analyses during patent examination. On Monday, we reported on the opening remarks provided by Drew Hirshfeld, Deputy Commissioner for Patent Examination Policy, and the overview of the Guidance provided by Raul Tamayo, Senior Legal Advisor for the Office of Patent Legal Administration. Today, we report on the comments provided by several of the public presenters.
As we noted in Part I, the ten public presenters were divided into three groups, with each presenter being given 10 minutes to provide feedback on the Guidance. The first group of presenters consisted of Dr. Hans Sauer, Deputy General Counsel for Intellectual Property for the Biotechnology Industry Organization; Suzannah K. Sundby of Smith, Gambrell & Russell, LLP; Dr. Anthony D. Sabatelli of Dilworth IP LLC; and Dr. Kenneth H. Sonnenfeld of King & Spalding LLP.
 Dr. Sauer (at left) began by noting that "BIO member companies operating in areas that have little or nothing to do with human genetics are beginning to receive 101 rejections of claims to pharmaceutical compositions, industrial enzymes, methods of treatment using medicinal molecules, and other inventions that were neither considered nor discussed in recent Supreme Court decisions." He pointed out that BIO members believe that in crafting the Guidance, "the PTO has gone beyond interpretation of recent Supreme Court decisions, and has engaged in unwarranted extrapolation and expansion of law, in ways not prompted by the Myriad decision." According to Dr. Sauer, notable examples of this expansion include "the application of a new 101 rationale to combination products," methods of drug administration or treatment, and "[t]he implicit but clear abandonment of cases like Parke-Davis, In re Merz, In re Bergstrom, In re Kratz, and Merck v Olin Mathieson, which were briefed to the Supreme Court but neither discussed nor overruled."
Dr. Sauer (at left) began by noting that "BIO member companies operating in areas that have little or nothing to do with human genetics are beginning to receive 101 rejections of claims to pharmaceutical compositions, industrial enzymes, methods of treatment using medicinal molecules, and other inventions that were neither considered nor discussed in recent Supreme Court decisions." He pointed out that BIO members believe that in crafting the Guidance, "the PTO has gone beyond interpretation of recent Supreme Court decisions, and has engaged in unwarranted extrapolation and expansion of law, in ways not prompted by the Myriad decision." According to Dr. Sauer, notable examples of this expansion include "the application of a new 101 rationale to combination products," methods of drug administration or treatment, and "[t]he implicit but clear abandonment of cases like Parke-Davis, In re Merz, In re Bergstrom, In re Kratz, and Merck v Olin Mathieson, which were briefed to the Supreme Court but neither discussed nor overruled."
Because "[t]he PTO is merely interpreting court decisions interpreting prior court decisions interpreting judicially-created exceptions from the statute," Dr. Sauer contended that "the PTO is no better positioned to establish an authoritative interpretation of the Supreme Court’s 101 jurisprudence than are the lower courts." He added that:
Expanding Myriad's holding to all claims to isolated or purified natural molecules like antibiotics and other medicinal substances, and combinations thereof, and fermentation or distillation products or bacterial enzymes, will not only prospectively block inventors from acquiring commercially meaningful protection for products that were never even mentioned by the Supreme Court [but will] also cast[] a shadow over thousands of issued patents that the PTO now says would never be issued if they were examined today and -- implicitly -- should never have been issued in the first place.
Dr. Sauer also noted that "[t]he PTO's interpretation of the Supreme Court cases, as applied to biotech, is not the only permissible one," and then set forth a series of questions:
So what policy choice did the PTO make when it picked its current interpretation from all the different reasonable interpretations of these cases? Does the PTO believe the Supreme Court really meant to strike down claims to fungal antibiotics or industrial enzymes that go into laundry detergents? Does the PTO believe it is implementing the intent of Congress when it last passed Section 101? Or that a more draconian interpretation of Supreme Court pronouncements will advance the progress of the useful arts better than alternative readings?
As for answers to these questions, Dr. Sauer responded that "[w]e're left to guess, because that's a discussion we never had." He concluded his comments by declaring that:
There is no unified reading of Funk, Benson, Flook, Diehr, Chakrabarty, Pioneer, Bilski, Mayo, and Myriad that is fully coherent, free of internal tension, and that operates harmonically with the other requirements of patentability. We need the PTO to acknowledge that reality. And we need a dialogue not only over how to best interpret the caselaw we've been given, but also whether that caselaw leads us to the right place.
A copy of Dr. Sauer's written testimony can be found here.
 The second presenter, Suzannah Sundby (at right), argued that there were three problems with the Guidance: (1) the factor-based approach set forth in the Guidance is inappropriate for § 101 eligibility determinations, (2) process and product claims should be evaluated using separate tests, and (3) the Office's interpretations of Mayo and Myriad in the Guidance are too broad. With respect to the first issue, Ms. Sundby indicated that "[a]n ineligible factor can negate an eligible factor," and as an example, pointed out that if the Guidance's weighing of factors approach was applied to the claims of Diehr, "the steps of installing the rubber in the press and the subsequent closing of the press would negate the fact that the process integrated the algorithm into a practical application, i.e., curing and molding rubber."
The second presenter, Suzannah Sundby (at right), argued that there were three problems with the Guidance: (1) the factor-based approach set forth in the Guidance is inappropriate for § 101 eligibility determinations, (2) process and product claims should be evaluated using separate tests, and (3) the Office's interpretations of Mayo and Myriad in the Guidance are too broad. With respect to the first issue, Ms. Sundby indicated that "[a]n ineligible factor can negate an eligible factor," and as an example, pointed out that if the Guidance's weighing of factors approach was applied to the claims of Diehr, "the steps of installing the rubber in the press and the subsequent closing of the press would negate the fact that the process integrated the algorithm into a practical application, i.e., curing and molding rubber."
As for the second issue, Ms. Sundby contended that the test for process claims should be the "significantly more" standard of Mayo, and the test for product claims should be the "markedly different characteristic" standard of Chakrabarty. Moreover, as she pointed out "[n]owhere does the [Supreme] Court hold that a product must be both significantly more and markedly different."
In a theme repeated by many of the presenters, Ms. Sundby reminded the Office that the standard set forth in Chakrabarty was whether a naturally occurring product recited in a claim exhibits markedly different characteristics, and not whether that product exhibits markedly different structure. She argued that this markedly different characteristic could be either a structural difference or a functional one, and that this interpretation would be consistent with both Funk Bros. and Myriad, where the patent ineligible products at issue did not possess new, different, or changed characteristics.
With respect to the Office's interpretations of Mayo and Myriad in the Guidance, Ms. Sundby contended that "the PTO seems to interpret the 'apply it' discussion in Mayo as requiring unduly specific process steps," and that the Office "appears to interpret Myriad as applying to all things, isolated or synthesized, that can be derived from a product of nature." According to Ms. Sundby, the Supreme Court in Myriad "reasoned that information in the isolated DNA molecule is the same as it is in nature, and hence, the chemical changes resulting from its isolation did not result in a markedly different characteristic, e.g., change to the information." She concluded, therefore, that "Myriad does not stand for the rigid proposition that all compositions and molecules that originate from products of nature are patent ineligible" (emphasis in presentation). As a result of the Office's interpretation of Myriad, many therapeutic compositions will be found to be patent ineligible (as outlined in a slide from her presentation):
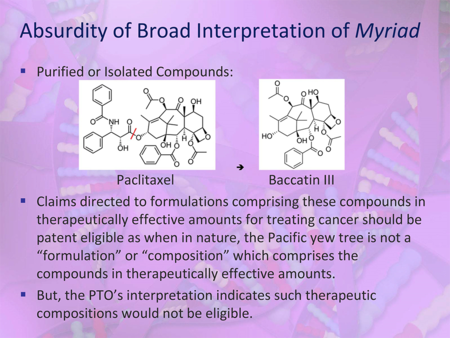
In concluding her presentation, Ms. Sundby suggested, inter alia, that the Office allow applicants of pending applications to switch elected inventions, make actual examples of claims and 101 analyses available to the public, and establish a procedure for reviewing eligibility determinations to ensure consistency and compact prosecution.
A copy of Ms. Sundby's presentation can be found here.
 The next presenter in the group, Dr. Sabatelli (at left), began by focusing on the problems raised by the pomelo juice example in the Office's training slides and the Office's comments regarding the patent eligibility of gunpowder -- a component of the claimed fountain-style firework of Example C in the Guidance. While acknowledging that there was a need for the Guidance and conceding that the Guidance was "an initial step in the right direction," he asserted that the Guidance was too complex and set forth too many factors (and suggested that more factors were likely to be added by the Office or the courts). He asked whether "we need[ed] a brighter, simpler line?" As for changes to the Guidance, Dr. Sabatelli asked the Office to provide more examples.
The next presenter in the group, Dr. Sabatelli (at left), began by focusing on the problems raised by the pomelo juice example in the Office's training slides and the Office's comments regarding the patent eligibility of gunpowder -- a component of the claimed fountain-style firework of Example C in the Guidance. While acknowledging that there was a need for the Guidance and conceding that the Guidance was "an initial step in the right direction," he asserted that the Guidance was too complex and set forth too many factors (and suggested that more factors were likely to be added by the Office or the courts). He asked whether "we need[ed] a brighter, simpler line?" As for changes to the Guidance, Dr. Sabatelli asked the Office to provide more examples.
A copy of Dr. Sabatelli's presentation can be found here.
 The final presenter in the first group, Dr. Sonnenfeld (at right), began his presentation by a showing a slide listing the claims of Example B of the Guidance:
The final presenter in the first group, Dr. Sonnenfeld (at right), began his presentation by a showing a slide listing the claims of Example B of the Guidance:
Claim 1: Purified amazonic acid.
Claim 2: Purified 5-methyl amazonic acid.
Claim 3: A method of treating colon cancer, comprising: administering a daily dose of purified amazonic acid to a patient suffering from colon cancer for a period of time from 10 days to 20 days, wherein said daily dose comprises about 0.75 to about 1.25 teaspoons of amazonic acid.
He argued that Example B created gaps in the available protection for many biotech and pharmaceutical applicants. He noted, for example, that pharmaceutical composition claims (for which no exemplary claim was set forth in the Guidance) may be too narrow if a pharmaceutically acceptable carrier must impart a "significant difference," and that method of treatment claims could be limited to single indications and require the inclusion of multiple limiting elements. With respect to claim 1 of Example B, he asked whether the Office was analyzing the claim as a whole, and suggested that a claim to "amazonic acid" was different from a claim to "purified amazonic acid" (stating that purified amazonic acid does not exist in nature). In support for the patent eligibility of purified natural product claims, Dr. Sonnenfeld pointed to the Office's utility guidelines (as shown in the slide from his presentation):

And provided a list of patented purified natural product therapeutics:
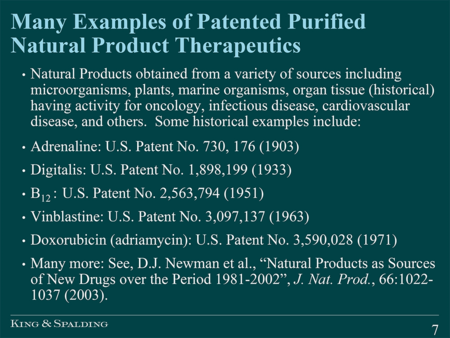
Like Ms. Sundby, Dr. Sonnenfeld also argued that Myriad concerns genes and their information, and that the Myriad holding is limited to genes:

A copy of Dr. Sonnenfeld's presentation can be found here.
Before moving on to the second group of presentations, representatives from the Office fielded questions and received additional comments from attendees. One attendee remarked that the Guidance had been a "process failure" because the Office had neglected to include the community of practitioners sooner. In response, Mr. Hirshfeld stated that it was not the Office's intention to exclude the public from the discussion, but that the Office, in crafting the Guidance, was merely doing what it always had done with respect to the creation of examination guidelines. When Office representatives were asked by another attendee whether there was a Supreme Court case that said "markedly different" was the test for determining the eligibility of claims reciting natural products, Mr. Tamayo responded that the Office believed "Myriad focused us on that being the test." The forum then moved on to the second group of presenters.
In subsequent posts, we will examine the comments made by the remaining public presenters as well as the open participation/question and answer session that concluded the forum.
For additional information regarding this topic, please see:
• "Guest Post: How to Patent Grapefruit Juice -- The New USPTO Guidance for Patent Eligible Subject Matter Is Both Sticky and Sour," May 13, 2014
• "USPTO Holds Forum on Subject Matter Eligibility -- Part I," May 12, 2014
• "USPTO Tries to Address Public Misunderstandings Regarding Myriad-Mayo Guidance," April 16, 2014
• "USPTO Issues Guidance for Analyzing Subject Matter Eligibility of Claims Reciting Laws of Nature/Natural Principles, Natural Phenomena or Natural Products," March 4, 2014