
The U.S. Food and Drug Administration (FDA) has approved Abbott’s Tecnis Symfony Intraocular Lenses (IOL) for treating cataracts. According to Abbott, the Tecnis Symfony lenses are currently the only lenses in the United States that “provide a full range of continuous high-quality vision following cataract surgery, while also mitigating the effects of presbyopia by helping people focus on near objects.” Outside the United States, Abbot states that the Symfony lens is approved in more than 50 countries and has been implanted in more than 2,000 eyes as part of clinical studies.
Cataracts are a medical condition in which clouding of the eye’s lens affects vision. The condition becomes more common with increasing age. In fact, the National Eye Institute explains that a majority of Americans have cataracts or have undergone cataract surgery by the age 80.
Surgeons can replace the natural lens of a patient’s eye with an IOL implant, such as a monofocal lens, during a cataract surgery. However, while monofocal lenses can improve patients’ long distance vision, they are less effective at improving short range vision. Abbott uses its proprietary diffractive echelette feature to improve short range vision (see illustration below).
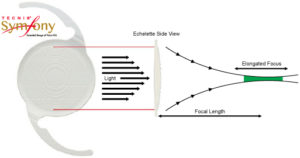
According to
Thomas Frinzi,
Senior Vice President of Abbott’s vision business:
Abbott is focused on improving people’s vision and their lives by helping them stay healthy and active. Symfony offers patients, including those with astigmatism, an option for crisp, clear vision at all distances. This is an important addition to our portfolio of lenses, as we expect many patients to choose a Symfony lens over a standard monofocal lens, given its benefits. We are happy that we can offer more people around the world this new category of lenses.
The FDA approval also includes Abbott’s Tecnis Symfony Toric IOL for patients having astigmatism.
Graph Source: Abbott Medical Optics